This article on organic reactions is a special one in our organic chemistry series. It will guide you through how chemical transformations unlock diversity at the molecular level.
You will find it one of the most comprehensive sources on named reactions in organic chemistry available on the web.
Moreover, we have also included a cheat sheet for you at the end of this article so that you can have a road map to learn organic chemistry in the most fun way possible.
Thus, without any further ado, let’s start reading!
List of organic chemistry reactions
Reactions of alkanes
- Halogenation reaction of alkanes
Substrate: Saturated hydrocarbons/alkanes (CH4, C2H6, C3H8, etc.)
Reagent: Halogen X2 (F2, Cl2, Br2, I2)
Conditions: UV-light, 25°C temperature or Heating at (250-400) °C
Mechanism: Free radical substitution
Order of reactivity:
The reactivity of halogens follows the order: F2 >> Cl2 > Br2 > I2
Product: Halogenoalkane or alkyl halide (R-X)

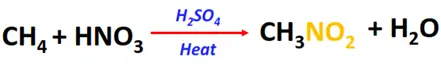
- Nitration of alkanes
Substrate: Saturated hydrocarbons/alkanes (CH4, C2H6, C3H8, etc.)
Reagent: Nitric acid (HNO3)
Conditions: Excess of 60% HNO3 at 400° C temperature
Product: Nitroalkane (R-NO2)
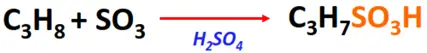
- Sulfonation of alkanes
Substrate: Saturated hydrocarbons/alkanes (CH4, C2H6, C3H8, etc.)
Reagent & Conditions: H2SO4 + SO3 or fuming sulfuric acid (H2S2O7)
Product: Alkane sulfonic acid (R-SO3H)
- Combustion reaction of alkanes
Substrate: Saturated hydrocarbons/alkanes (CH4, C2H6, C3H8, etc.)
Reagent & Conditions: Ignition at very high temperatures in the presence of air or oxygen
Products: Carbon dioxide (CO2) + water (H2O)

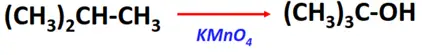
- Oxidation reaction of alkanes
Substrate: Alkanes containing tertiary hydrogen (R2-CH-R)
Reagent: Oxidizing agent (KMnO4)
Mechanism: Addition of oxygen
Products: Tertiary alcohol
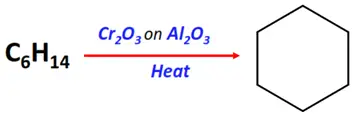
- Hydroforming or catalytic reforming of alkanes
Substrate: Long-chain alkanes (such as n-hexane)
Reagent & Conditions: Heating at 450-550 °C under pressure in the presence of chromic oxide supported on Al2O3.
Mechanism: Hydrogenation, cyclization followed by isomerization
Product: Aromatic compound (such as benzene from n-hexane)
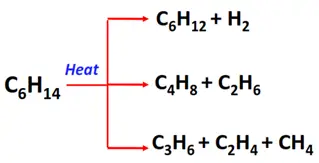
- Pyrolysis or cracking of alkanes
Substrate: Long-chain alkanes
Condition: Heating at high temperatures (500°C)
Mechanism: Thermal decomposition
Products: A mixture of short-chain alkanes and alkenes
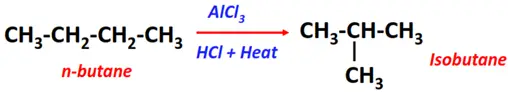
- Isomerization of alkanes
Substrate: Alkane
Reagents: HCl
Conditions: AlCl3 at 300°C temperature
Products: Isomer of the parent molecule
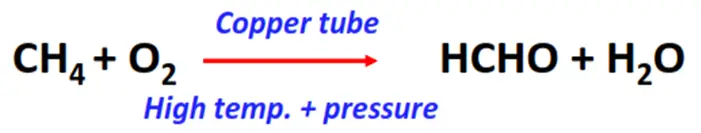
- Oxidation of methane
Substrate: Methane (CH4)
Reagents & Conditions: Copper tube, 200°C temperature, 100 atm pressure
Product: Formaldehyde (HCHO)
Reactions of alkenes
- Hydrogenation of alkenes
Substrate: Unsaturated hydrocarbon/alkenes (C2H4, C3H6, C4H8)
Reagent: Hydrogen (H2) gas
Conditions: Pt, Pd or Ir catalyst in finely divided form or Raney Nickel
Mechanism: Electrophilic addition reaction
Product: Saturated hydrocarbons (alkanes)

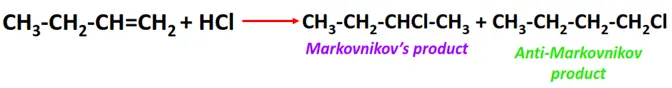
- Addition of hydrogen halides to alkenes
Substrate: Unsaturated hydrocarbon/alkenes (C2H4, C3H6, C4H8)
Reagent: Hydrogen halide (HX)
Conditions: Passing dry gaseous HX directly through the alkene sample
Mechanism: Electrophilic addition reaction (involving a carbocation intermediate)
Product: Alkyl halide (R-X)
Order of reactivity:
The reactivity of hydrogen halide follows the order: HI > HBr > HCl > HF
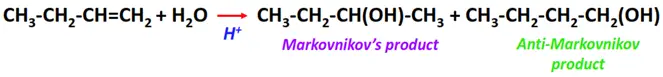
- Hydration of alkenes
Substrate: Unsaturated hydrocarbon/alkenes (C2H4, C3H6, C4H8)
Reagent: Water (H2O)
Conditions: Acidic conditions
Mechanism: Electrophilic addition reaction
Product: Alcohol (R-OH)
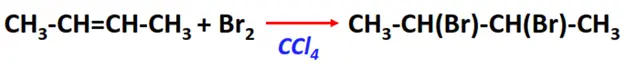
- Addition of halogens
Substrate: Unsaturated hydrocarbon/alkenes (C2H4, C3H6, C4H8)
Reagent: Halogen (Cl2, Br2, etc)
Conditions: Inert solvent (such as CCl4) at room temperature
Mechanism: Electrophilic addition reaction (a cyclic halonium ion is formed as an intermediate)
Product: Halogenoalkane (vic-dichlorides or vic-dibromides)
- Epoxidation reaction
Substrate: Unsaturated hydrocarbon/alkenes (C2H4, C3H6, C4H8)
Reagent: Peracetic acid
Mechanism: Electrophilic addition
Product: Cyclic ether
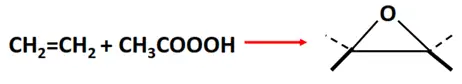
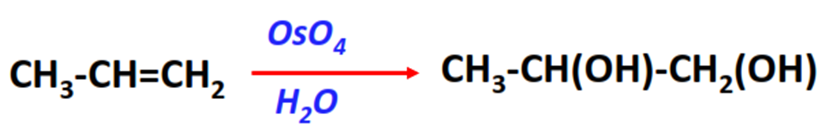
- Hydroxylation reaction
Substrate: Unsaturated hydrocarbon/alkenes (C2H4, C3H6, C4H8)
Reagents: Osmium tetrachloride (OsO4) and H2O
Mechanism: Oxidation (addition of OsO4 followed by hydrolysis with water)
Product: Syn-diol
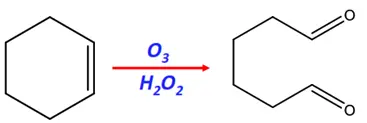
- Ozonolysis
Substrate: Cyclic alkenes (such as cyclohexene)
Reagents: Ozone (O3) gas and hydrogen peroxide (H2O2)
Mechanism: Oxidation
Product: Dioic acid (an Ozonide)
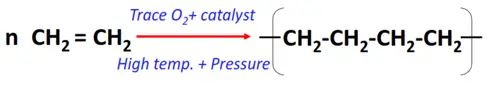
- Polymerization reaction of alkenes
Substrate: A large number of alkene monomers
Mechanism: Chain addition of monomers
Product: Polymer
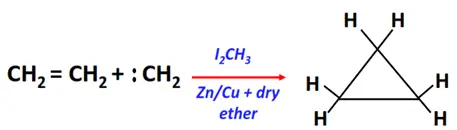
- Addition of carbenes (Simmon Smith reaction)
Substrate: Unsaturated hydrocarbon/alkenes (C2H4, C3H6, C4H8)
Reagent & Conditions: Simmon Smith reaction mixture (I2CH3 and Zn/Cu couple in anhydrous ether)
Mechanism: Electrophilic addition via a carbene (CR2) intermediate
Product: Cycloalkane
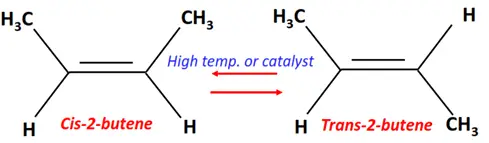
- Isomerization of alkenes
Substrate: Unsaturated hydrocarbon/alkenes (C2H4, C3H6, C4H8)
Reagent & Conditions: Very high temperature (500-700°C) or low temperature in the presence of an AlCl3 catalyst
Product: Isomer of the parent molecule
Reactions of alkyl halides
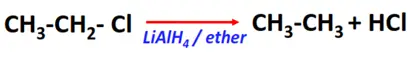
- Reduction reaction
Substrate: Alkyl halides (R-Cl, R-Br, R-I)
Reagent & Conditions: Reducing agents (LiAlH4 or NaBH4) in ether
Mechanism: Hydride transfer
Product: Alkane (R-H)
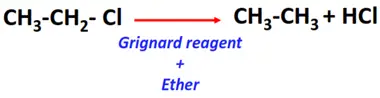
- Catalytic hydrogenolysis of alkyl halides
Substrate: Alkyl halides (R-Cl, R-Br, R-I)
Reagent & Conditions: Grignard reagent (R-Mg-X) prepared in ether
Mechanism: Hydrolysis
Product: Alkane (R-H)
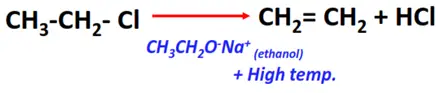
- Dehydrohalogenation of alkyl halides
Substrate: Alkyl halides (R-Cl, R-Br, R-I)
Reagents: Sodium ethoxide (CH3CH2O–Na+) prepared in ethanol (CH3CH2OH)
Condition: High temperature (300-400°C)
Mechanism: Elimination
Product: Alkene (R-C=C-R)
- Dehalogenation of vic-dihalides
Substrate: Vic-dihalide (Cl-R-R-Cl, Br-R-R-Br)
Reagent & Conditions: Zinc dust in an anhydrous solvent (CH3OH or CH3COOH)
Mechanism: Elimination
Product: Alkene (R-C=C-R)

Reactions of alcohols
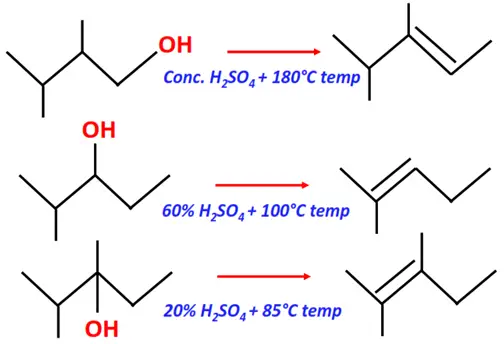
- Dehydration of alcohols
Substrate: Alcohol (primary, secondary or tertiary)
Reagent: Sulfuric acid (H2SO4)
Conditions:
- Conc. H2SO4, 180° C temperature for primary alcohols
- 60-80 % H2SO4, 100°C for secondary alcohols
- 20 % H2SO4, 85 °C for tertiary alcohols
Mechanism: Elimination
Product: Alkene (R-C=C-R)
- Reaction of alcohols with metals
Substrate: Alcohol (R-OH)
Reagent: Sodium (Na) metal
Products: Sodium alkoxide (R-O–Na+) and hydrogen (H2)
Order of reactivity: The reactivity of alcohols decreases in the order: primary > secondary > tertiary.

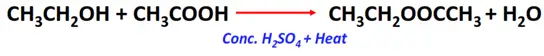
- Esterification reaction of alcohols with carboxylic acids
Substrate: Alcohol (R-OH)
Reagent: Carboxylic acid (R-COOH)
Conditions: Acidic (conc. H2SO4) + heat
Mechanism: Condensation reaction
Products: Sodium alkoxide (R-O–Na+) and water (H2O)
- Oxidation of primary alcohols
Substrate: Primary alcohol (R-CH2-OH)
Reagent & Conditions: Acidified oxidizing agent (K2Cr2O7/H+) + heat
Mechanism: Addition of oxygen
Products: Aldehyde (R-CHO) under controlled conditions followed by carboxylic acid (R-COOH) after complete oxidation.
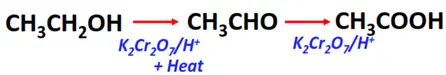
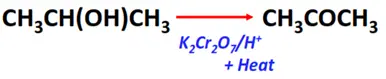
- Oxidation of secondary alcohols
Substrate: Secondary alcohol (R-CH(OH)-R)
Reagent & Conditions: Acidified oxidizing agent (K2Cr2O7/H+) + heat
Mechanism: Removal of hydrogen
Product: Ketone (R-CO-R)
Reactions of aldehydes and ketones
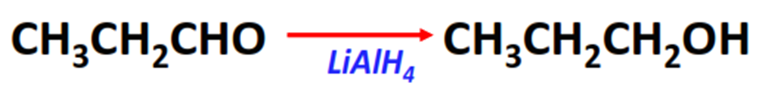
- Reduction reaction of aldehydes
Substrate: Aldehyde (R-CHO)
Reagent: Reducing agents (such as LiAlH4)
Mechanism: Nucleophilic addition
Product: Primary alcohol (R-CH2-OH)
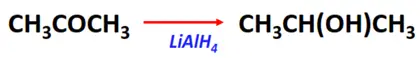
- Reduction reaction of ketones
Substrate: Ketone (R-CO-R)
Reagent: Reducing agents (such as LiAlH4)
Mechanism: Nucleophilic addition
Product: Secondary alcohol (R-CH(OH)-R)
Important named reactions in organic chemistry
An organic reaction is usually named on the name of the scientist who introduced it. Given below are some popular named reactions in organic chemistry. These are mentioned in alphabetical order.
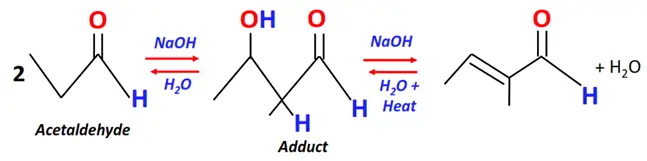
- Aldol condensation
Substrate: 2 equivalents of carbonyl compounds (aldehydes or ketones having one or more alpha hydrogens) such as acetaldehyde.
Reagent & Conditions: NaOH + H2O + Heat
Mechanism: Nucleophilic addition, an enolate is formed as an intermediate
Product: Aldol (possessing both aldehyde (CHO) and alcohol (OH) functional groups)
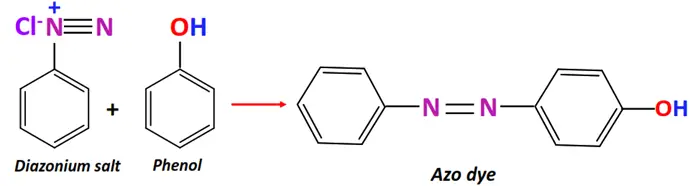
- Azo coupling
Substrate: Diazonium salt (Ar-N≡N-X) + aromatic compound (Ar’-H) such as phenol or aniline
Reagent & Conditions: Mildly alkaline solution
Mechanism: Electrophilic aromatic substitution
Product: Azo dye
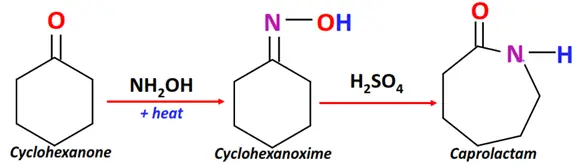
- Beckmann rearrangement
Substrate: Cyclohexanone + hydroxylamine
Reagent & Conditions: Acidic conditions +heat
Mechanism: Rearrangement reaction that proceeds via oxime formation
Product: Cyclic amide (such as Caprolactam)
- Cannizzaro’s reaction
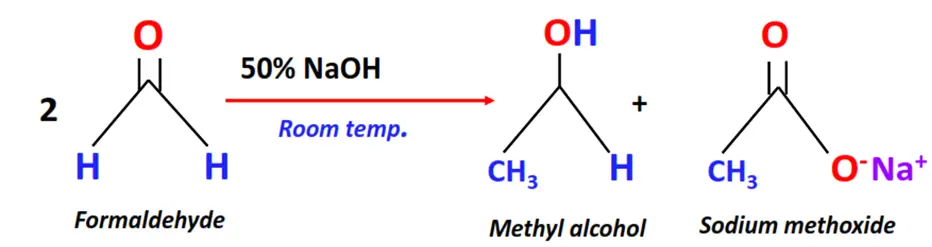
Substrate: 2 equivalents of aldehydes containing no alpha hydrogen, such as formaldehyde
Reagent & Conditions: 50% aq. or alcoholic NaOH at room temperature
Mechanism: Self-oxidation followed by reduction of the aldehyde
Products: Alcohol + Metal oxide
- Claisen-Schmidt condensation
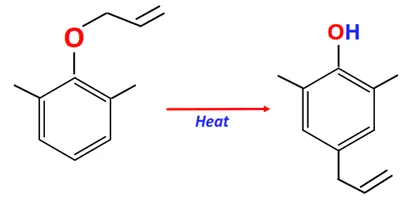
Substrate: Phenolic allyl ether
Conditions: Heat
Mechanism: Molecular rearrangement via [3,3]-sigmatropic shift
Product: 2-allyl phenol
The Claisen-Schmidt condensation is also known as crossed aldol condensation.
- Clemmensen reduction
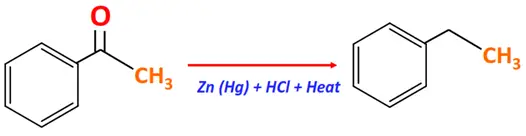
Substrate: Carbonyl compound (Aldehyde or Ketone)
Reagent & Conditions: Zinc amalgam in hydrochloric acid
Mechanism: Removal of oxygen and addition of hydrogen, zinc organyl formed as an intermediate
Product: Alkane
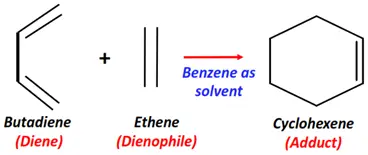
- Diels-Alder reaction
Substrate: Diene + Dienophile (alpha, beta-unsaturated carbonyl compound)
Reagent & Conditions: Benzene + 20°C temperature
Mechanism: Cycloaddition
Product: Adduct (six-membered ring)
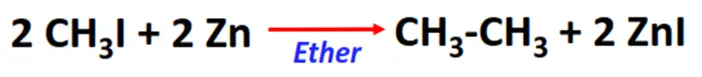
- Frankland reaction
Substrate: Iodomethane (CH3I)
Reagent & Conditions: Zinc (Zn) metal and ether
Product: Ethane (CH3-CH3)
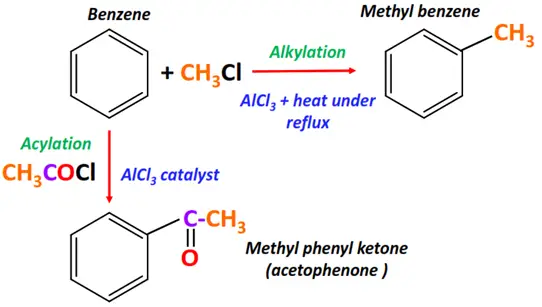
- Friedel craft reaction
The Friedal craft catalyst is a Lewis acid (AlCl3) used for the following two types of coupling reactions:
1. Friedel craft alkylation
Substrate: Benzene
Reagent: Alkyl halide (R-X)
Conditions: AlCl3 catalyst, reflux anhydrous conditions
Mechanism: Electrophilic aromatic substitution
Product: Alkyl benzene
2. Friedel craft acylation
Substrate: Benzene
Reagent: Acyl halide (R-(C=O)-X)
Conditions: AlCl3 catalyst
Mechanism: Electrophilic aromatic substitution
Product: Alkyl phenyl ketone
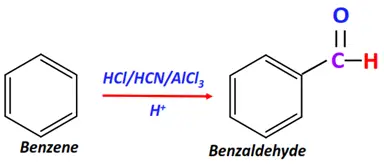
- Gattermann reaction
Substrate: Benzene
Reagents: A mixture of hydrogen cyanide (HCN) and hydrochloric acid (HCl)
Conditions: AlCl3 catalyst
Mechanism: Electrophilic aromatic substitution
Product: Benzaldehyde
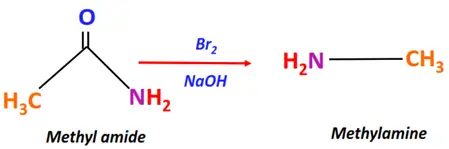
- Hofmann rearrangement
Substrate: Amide (R-CONH2 or Ar-CONH2)
Reagent & Conditions: Br2 + NaOH
Mechanism: Rearrangement reaction via an isocyanate intermediate
Product: Amine (R-NH2 or Ar-NH2)
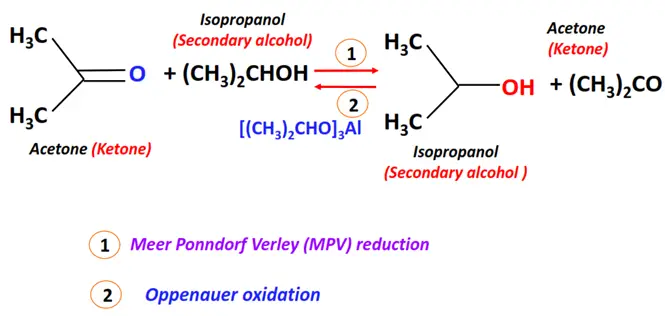
- Meerwein-Ponndorf-Verley (MPV) reduction
Substrate: Aldehyde or Ketone
Reagent & Conditions: Alcohol (such as isopropanol) and aluminum alkoxide (Al(OPri)3)
Mechanism: Pericyclic (hydride transfer)
Product: Primary alcohol (for aldehyde)/ Secondary alcohol (for ketone)
- Oppenauer oxidation
Oppenauer oxidation is the reverse of MPV reduction.
Substrate: Alcohol
Reagent & Conditions: Aluminium alkoxide (Al(OPri)3)
Mechanism: Pericyclic
Product: Carbonyl compound (aldehyde or ketone)
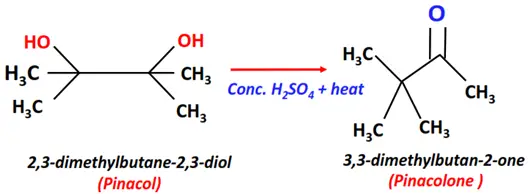
- Pinacol-pinacolone rearrangement
Substrate: Dihydric alcohol, i.e., Pinacol (2,3-dimethylbutan-2,3-diol)
Reagent & Conditions: Conc. H2SO4 + Heat
Mechanism: Rearrangement
Product: Pinacolone (3,3-dimethylbutan-2-one)
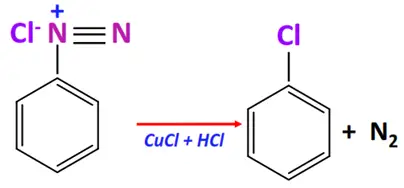
- Sandmeyer’s reaction
Substrate: Benzene diazonium salt (ArN2+X–)
Reagent & Conditions: Cu-X’ + HCl or CuCN + KCN at room temperature
Mechanism: Replacement of the diazonium group by another group
Product: Ar-X’ or Ar-CN
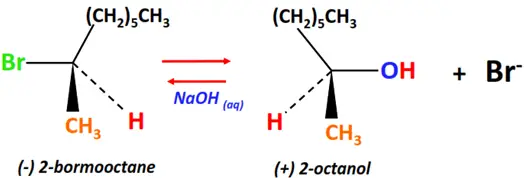
- Walden inversion
Walden inversion refers to any chemical reaction that yields a product whose stereochemical configuration is opposite to that of the reactant molecule.
For example;
Substrate: levorotatory-2-bromooctane
Reagent & Conditions: Alkaline (NaOH)
Mechanism: Nucleophilic substitution (SN2)
Product: dextrorotatory-2-Octanol
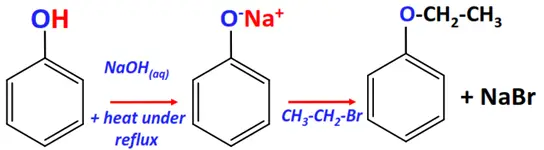
- Williamson ether synthesis
Substrate: Phenol (Ar-OH)
Reagent: Alkyl halide (R-X)
Conditions: Aqueous NaOH + heat under reflux for 3 hrs.
Mechanism: Nucleophilic substitution (SN2)
Product: Ether (Ar-O-R)
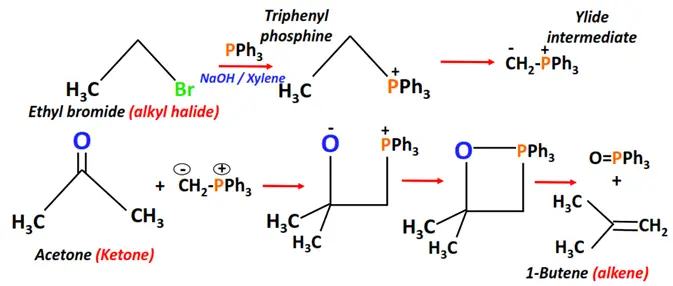
- Witting reaction
Substrate: Aldehyde or Ketone
Reagents: Alkyl halide (R-X) + Triphenylphosphine (PPh3)
Conditions: NaOH in an inert solvent such as xylene
Mechanism: Nucleophilic addition via a ylide intermediate
Product: Alkene
- Wurtz-Fitting reaction
Substrate: Iodomethane (CH3I)
Reagent & Conditions: Sodium (Na) metal and ether
Product: Ethane (CH3-CH3)
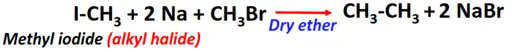
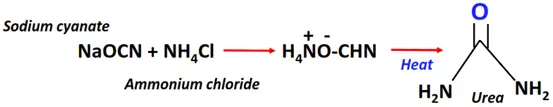
- Wohler synthesis
The Wohler synthesis is often cited as the foundation stone for organic chemistry.
Reagent & Conditions: A mixture of sodium cyanate (NaCNO) and ammonium chloride (NH4Cl) is heated and then cooled to produce ammonium cyanate
Mechanism: Proton transfer to ammonium cyanate followed by tautomerism
Product: Urea (H2N-C(=O)-NH2)
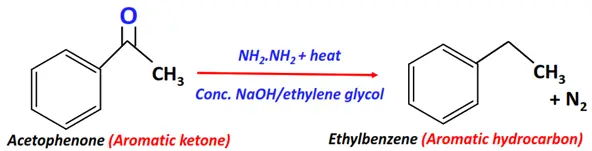
- Wolff-Kisnher reduction
Substrate: Aromatic ketone (Ar-C(=O)-R)
Reagent: Reducing agent, i.e., hydrazine (NH2NH2)
Conditions: Strong base (conc. KOH) prepared in ethylene glycol (HO-(CH2)2-OH) + heat
Mechanism: Reduction via a semicarbazone intermediate
Products: Aromatic hydrocarbon (Ar-H) + Nitrogen (N2)
For a detailed study of the above-mentioned reaction mechanisms, you may consult our article: 6 main types of reactions in organic chemistry.
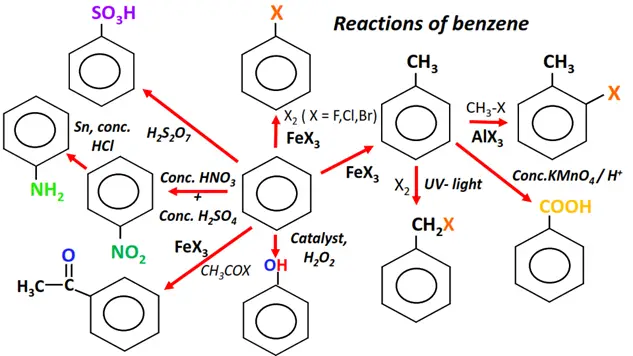
Meanwhile, the cheat sheet provided below shows the important reactions of benzene, the parent member of the aromatic family.
Also, check out this other wonderful source on organic reactions and mechanisms.
You may also want to learn some tips and tricks on studying organic chemistry.
Last but not least, we would like you to test your knowledge on organic reactions via this very helpful exercise.
