Carboxylic acids and esters are a part of our everyday life. Acetic acid in vinegar, ascorbic acid (Vitamin C) in oranges, citric acid in lemon and lime, and acetylsalicylic acid present in aspirin are all examples of carboxylic acids. In contrast, esters are carboxylic acid derivatives that give a characteristic aroma to fruits, food flavorings, perfumes, essential oils, etc.
If you are keen to learn what are carboxylic acids and esters from the lens of an organic chemist, then continue reading the article.
In this article, we have discussed what are carboxylic acids and esters, their structure, properties, naming, uses, reactions and preparation.
What is a carboxylic acid? – Definition, Structure & Examples
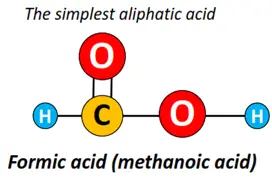
Carboxylic acids are organic compounds containing a carboxyl (COOH) functional group. The COOH group may be attached to an H-atom (HCOOH), an aliphatic (acyclic) hydrocarbon chain (R-COOH) or to an aryl ring (Ar-COOH). The general formula for a carboxylic acid molecule is CnH2n+1COOH.
Methanoic acid, popularly known as formic acid (HCOOH), is the simplest carboxylic acid molecule.
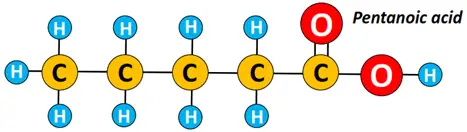
However, ethanoic acid or acetic acid (CH3COOH) is the most common carboxylic acid, followed by propanoic acid, butanoic acid, pentanoic acid, etc., all members of the big aliphatic carboxylic acid family.
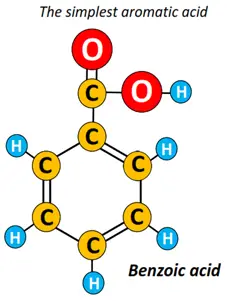
In contrast, benzoic acid (C6H5COOH) is the simplest aromatic carboxylic acid.
What is an ester? – Definition, Structure & Examples
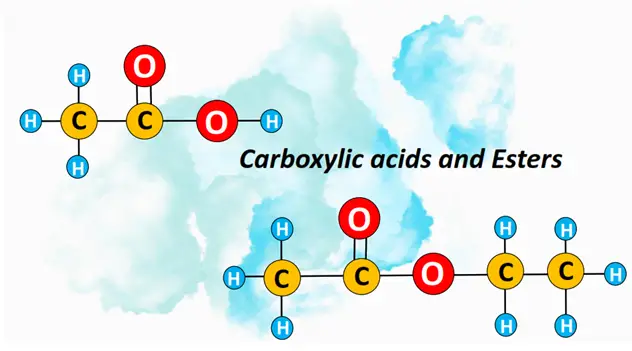
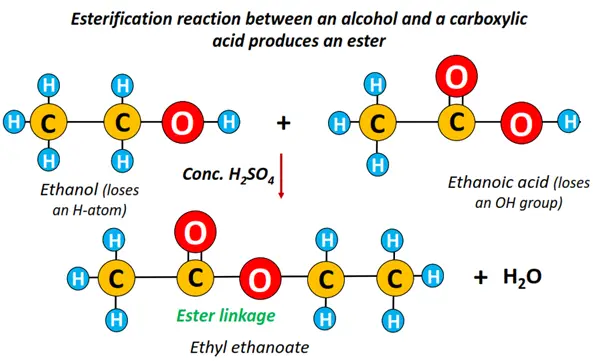
Esters are derivatives of carboxylic acid, defined by an ester (COO) linkage. The condensation reaction of an alcohol with a carboxylic acid produces an ester by losing a water molecule. This chemical reaction is known as esterification. Esters are represented by a general formula CnH2n+1COOCnH2n+1 or R-COO-R’ where R and R’ denote alkyl chains attached on either side of the ester linkage.
An example of an ester is ethyl ethanoate (CH3COOCH2CH3), formed by the esterification of ethanoic acid and ethanol. Other examples of esters include propyl ethanoate and butyl propanoate, etc.
Carboxylic acids and Esters: Nomenclature
The root name alkanoic acid is used for carboxylic acids.
For instance, a straight-chain carboxylic acid molecule containing 2 C-atoms is called ethanoic acid (derived from ethane), while that containing 5 C-atoms is pentanoic acid (derived from pentane).
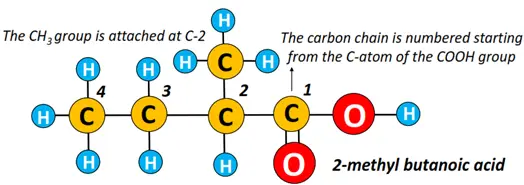
For branched molecules, the long carbon chain is numbered starting from the COOH-bonded carbon atom. The carboxylic acid is then named starting from the position of the substituent- substituent name followed by the name of the parent carboxylic acid. For e.g. 2-methyl-butanoic acid.
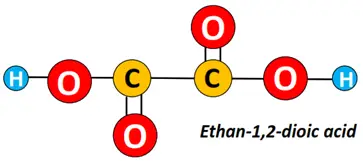
For a carboxylic acid containing two COOH groups, the name is given as;
name of the parent carbon chain- position of C-atoms carrying COOH groups followed by the term dioic acid.
An example includes pentane-1,5-dioic acid.
Oxalic acid is another example of a carboxylic acid possessing two COOH groups. Its IUPAC name is ethane-1,2-dioic acid.
On the other hand, the parent name for an ester is alkyl alkanoate.
Alkyl comes from carboxylic acid, while alkanoate is derived from the name of the alcohol used in the ester synthesis.
Hence, if an ester is synthesized by the esterification reaction of propanol with butanoic acid, its IUPAC name is then butyl propanoate. (Pro-tip: remember it’s not propyl butanoate).
Carboxylic acids and Esters: Properties
Carboxylic acids are:
- Liquids at r.t.p with sharp odors.
- Bronsted Lowry acids as they possess a proton (H-atom) available for donation. However, they are weak organic acids as they only partially ionize in water to liberate a limited number of H+ ions.
- Polar due to the presence of polar C-O (or C=O) and O-H bonds.
- Less polar than alcohols. Therefore, carboxylic acids possess higher boiling points as compared to alcohols having comparable molecular weight.
- Soluble in water, owing to their ability to form H-bonding with polar H2O molecules. However, increasing the length of the alkyl chain decreases the water solubility of the ester.
- Reactive with alcohols. However, this reactivity decreases with an increase in the number of C-atoms present in the alkyl chain.
Esters are:
- Liquids at r.t.p with a characteristic (usually sweet or pleasant) smell.
- Derivatives of carboxylic acids and alcohols.
- Polar and thus volatile in nature. But less polar than carboxylic acids and thus have lower melting and boiling points than the former.
- Slightly water-soluble.
Preparation and reactions of carboxylic acids and esters
Carboxylic acids are generally prepared by the following chemical reactions:
- Oxidation of primary alcohols
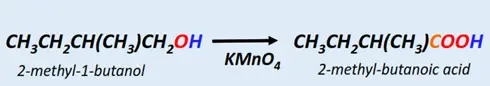
The complete oxidation of a primary alcohol (R-CH2-OH) produces a carboxylic acid (R-COOH). The oxidation is often carried out using an oxidizing agent such as potassium permanganate (KMnO4) or by using a catalyst.
Example: Oxidation of 2-methyl-1-butanol produces 2-methyl-butanoic acid.
Acetic acid, the most important carboxylic acid, is prepared by the catalytic oxidation of ethanol or acetaldehyde via atmospheric oxygen.
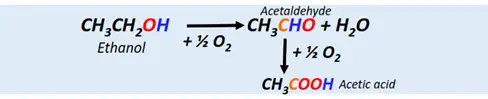
- Oxidation of alkyl benzenes
The alkyl (R) functional group attached to the benzene ring gets converted into COOH using KMnO4 or K2Cr2O7 under acidic conditions.
Example: Oxidation of toluene produces benzaldehyde followed by benzoic acid.
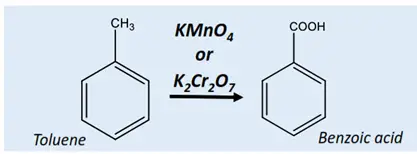
- Carbonation of Grignard’s reagent
An organomagnesium halide (having the general formula R-Mg-X) is referred to as the Grignard reagent. Bubbling carbon dioxide (CO2) through a solution of Grignard reagent prepared in ether yields a carboxylic acid molecule, as shown below.
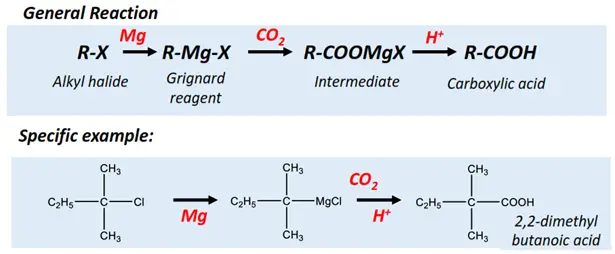
- Nitrile hydrolysis
The hydrolysis of a nitrile (R-CN) under acidic conditions produces a carboxylic acid (R-COOH) molecule of a corresponding chain length, while ammonia (NH3) is produced as a by-product.
Example: The hydrolysis of pentanenitrile produces pentanoic acid and ammonia.
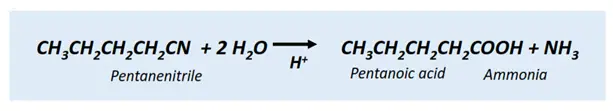
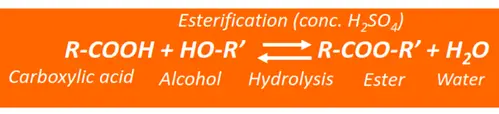
Preparation of esters from carboxylic acids (Esterification)
As mentioned earlier, an ester is prepared by heating an alcohol with a carboxylic acid using conc. sulfuric acid (H2SO4), as shown below.
The H is removed from the alcohol molecule, while the OH is removed from a carboxylic acid. The H-atom combines with OH to form H2O. As a result, an ester (-COO-) linkage is formed.
Ester hydrolysis is the reverse of esterification. The acidic hydrolysis of an ester produces carboxylic acid and an alcohol, while its basic hydrolysis yields alcohol and a carboxylate ion, respectively.
Some important chemical reactions of carboxylic acids are as discussed below:
- Reaction with metals
Like mineral acids, carboxylic acids react with metals to form metal carboxylate and release hydrogen (H2) gas.
Example: The reaction of ethanoic acid with zinc metal produces zinc ethanoate and hydrogen.

- Acid-base neutralization reaction
The acid-base neutralization reaction of a carboxylic acid with a base such as NaOH produces salt and water.
Example: The chemical reaction of ethanoic acid with sodium hydroxide produces sodium ethanoate and water.

A carboxylic acid molecule is first reduced to an aldehyde, followed by its complete reduction to primary alcohols.
Example: The reduction of propanoic acid using LiAlH4 produces propanal, followed by 1-propanol.

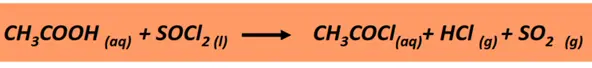
- Formation of an acyl halide from a carboxylic acid
Carboxylic acids react with thionyl chloride (SOCl2) or phosphorus pentachloride (PCl5) to form an acid chloride (R-CO-Cl).
Example: The chemical reaction of ethanoic acid with SOCl2 produces ethanoyl chloride while hydrogen chloride gas and sulfur dioxide are released as by-products.
What are carboxylic acids and esters used for – Applications
Carboxylic acids are used in:
- Medicinal drugs or pharmaceutical preparations such as acetylsalicylic acid, which is an integral component (API) of aspirin, the antihypertensive tablet.
- The chemistry laboratory as solvents.
- Soap manufacturing (saponification).
- Industrial synthesis, such as in the polymer industry.
Esters are used as:
- Flavoring agents.
- Primary constituents of fragrances, scented candles and other cosmetics.
- Monomer in polyester synthesis, which is then used for fiber making.
In a subsequent article, learn about other important uses and applications of organic compounds.
Do you know
Q.1) Which ester in bananas gives them their characteristic smell?
Answer: 3-methyl butyl ethanoate.
Q.2) Which instrumental analysis technique is used to separate and identify carboxylic acids and esters?
Answer: Gas chromatography coupled with mass spectroscopy (GCMS).
Find out more about GC and MS in the detailed articles provided by easytocalculate.com.
Also, you may like to practice some skill-building exercises on carboxylic acids and esters in this very informative article.
References
1. Barrett, A. G. M. 1991. 1.10 – Reduction of carboxylic acid derivatives to alcohols, ethers and amines. In: Trost, B. M. & Fleming, I. (eds.) Comprehensive organic synthesis. Oxford: Pergamon.
2. Kouichi, M., Rina, Y. & Yohei, O. 2018. Recent advances in the synthesis of carboxylic acid esters. In: Georgiana Ileana, B. & Gabriel Lucian, R (eds.) Carboxylic acid. Rijeka: Intech open.
3. Morrison, R. T., Boyd, R. N. & Bhattacharjee, S. K. 2013. Organic chemistry.