Phenols include the chemical compound phenol and its derivatives. Phenol is a versatile chemical compound, well-known as an essential ingredient in antiseptics and disinfectants, mouthwashes and lozenges.
So, without any further delay, let’s dive deep into the article and learn the chemistry, structure, properties, reactions, preparation and uses of phenols in detail.

Phenols: Definition & Structure
Phenols are organic compounds represented by a general formula ArOH. A hydroxyl (OH) functional group is directly attached to the phenyl ring in the phenol molecule.
If only one OH group is attached to an otherwise unsubstituted benzene ring, it is referred to as a specific compound, i.e., monohydroxybenzene, The phenol (C6H5OH). Other names for phenol are benzenol and carbolic acid.
In case of multiple substituents, other phenol derivatives are obtained, such as cresol, catechol, resorcinol, hydroquinone, etc. All these compounds, as a family, are known as phenols.

Properties of phenols
Phenols:
- Exist as either colorless liquids or white solids at r.t.p, possessing a low melting point (40.5°C).
- Possess a distinctive, sickeningly sweet, acrid odor.
- Possess high boiling points owing to their ability to form strong intermolecular hydrogen bonding.
- Are polar compounds that are highly water soluble.
- Are acidic in nature due to the presence of a proton donor OH group.
- Are flammable, so they can easily catch fire.
- Possess antioxidant and antiseptic properties.
Phenols nomenclature
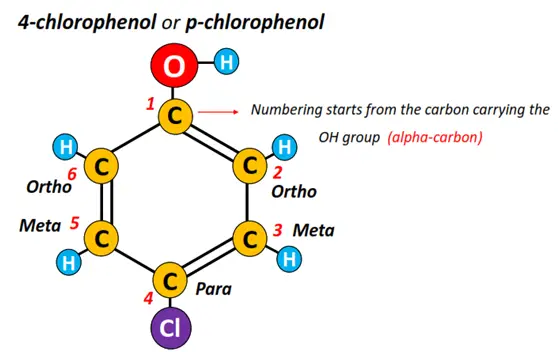
If a phenol molecule is substituted with additional groups, the molecule is named using the ortho, para, meta system w.r.t the parent OH group. The C-atoms are always numbered, starting from the carbon atom carrying the parent OH group. C-2 and C-6 are referred to as ortho positions, C-4 is para, while C-3 and C-5 are meta positions in the parent phenol molecule.
For example, in the molecule given below, a Cl-atom is attached at C-4 w.r.t the C-atom carrying the OH group. So, the compound’s name is para chloro phenol or p-chlorophenol.
Now let’s see another example. In this case, a nitro (NO2) group is attached at C-3 w.r.t, the C-atom carrying the OH group. Thus, the compound is named meta nitro phenol or m-nitrophenol, while its IUPAC name is 3-nitrophenol.

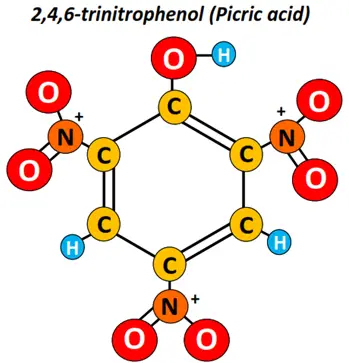
Similarly, in the tri-substituted phenol molecule shown below, three NO2 groups are occupying both the ortho and one para position. It is thus named 2,4,6-trinitrophenol (TNP). It is informally known as picric acid and is a well-known phenol, used as a raw material in explosive manufacturing. It was used for the first time in World War I.
Other commonly known phenols are:
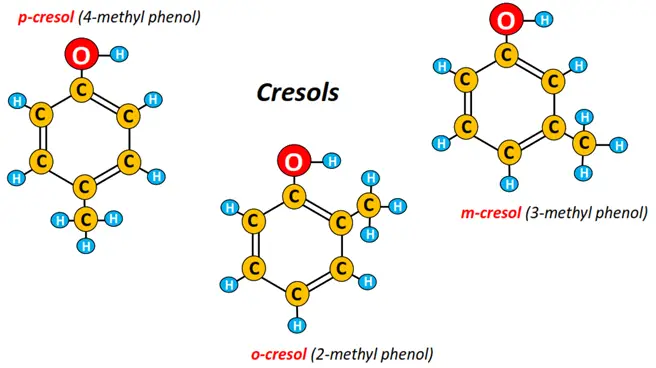
- Methyl phenols: Methyl phenols are known as cresols. A methyl (CH3) group can occupy ortho, para or meta positions to produce o-cresol (2-methyl phenol), p-cresol (4-methyl phenol) and m-cresol (3-methyl phenol), respectively.
- Hydroxyphenols: In hydroxyphenol molecules, two OH groups are directly attached to the phenyl ring. Any one OH group can be considered the parent hydroxyl group. If the other OH group is attached at C-2 w.r.t the C-atom carrying the parent OH group, then it is known as catechol (2-hydroxyphenol). In contrast, resorcinol is 3-hydroxyphenol, while hydroquinone is 4-hydroxyphenol.

Preparation of phenols
Phenols can be prepared by the following methods:
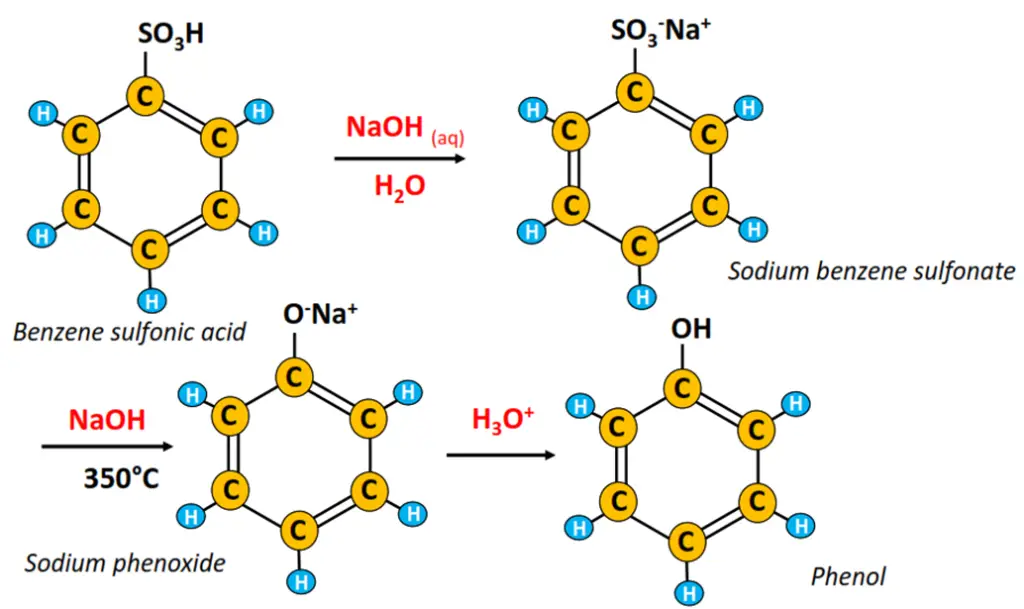
Pyrolysis
Heating benzene sulfonic acid with aq. NaOH produces sodium benzene sulfonate, which is, in turn, fused with solid NaOH at high temperatures to yield sodium phenoxide. As a last step, sodium phenoxide is acidified to produce phenol.
Oxidation of isopropyl benzene
It is also known as the cumene process, as it is used for the industrial production of phenol and acetone from cumene. Cumene (isopropyl benzene) is produced by the catalytic alkylation of benzene with propylene.

Cumene is then air oxidized to cumene hydroperoxide. The acidic cleavage of cumene hydroperoxide gives phenol and acetone. The cumene degradation proceeds following the carbocation mechanism (refer to the figure drawn below).

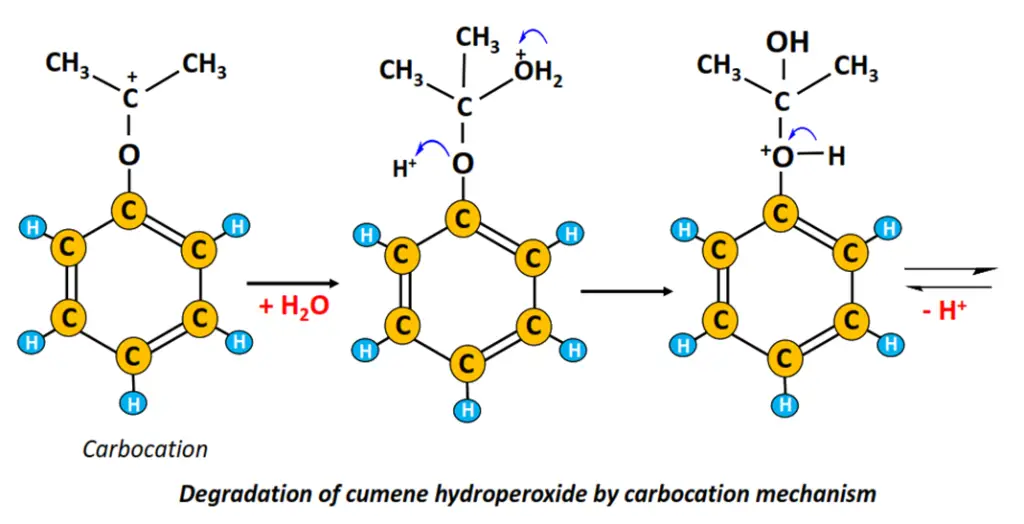

Dow process
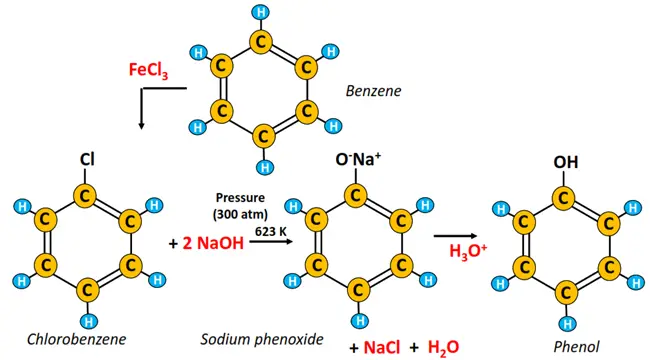
The Dow process of phenol synthesis was introduced by the Dow chemical company in the 1940s. It is based on the hydrolysis of chlorobenzene in the presence of a strong base (NaOH) at high temperatures.
The chlorination of benzene using a Lewis catalyst (FeCl3) produces chlorobenzene, which undergoes alkaline hydrolysis to produce sodium phenoxide, followed by its acidification to phenol.
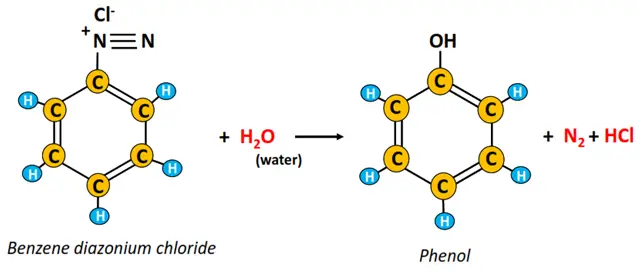
Hydrolysis of diazonium salts
The acidic hydrolysis of a diazonium salt such as benzene diazonium chloride produces phenol, while nitrogen (N2) and hydrogen chloride (HCl) gases are released as by-products.
Reactions of phenols
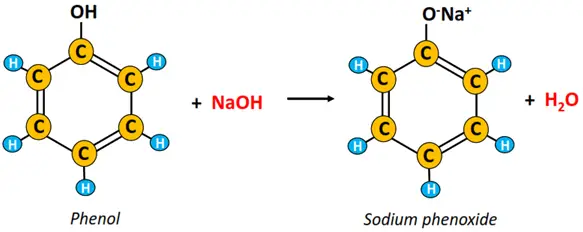
i) Acid-base neutralization reaction
Phenol reacts with a strong base, such as sodium hydroxide (NaOH), to produce sodium phenoxide (salt) and water.
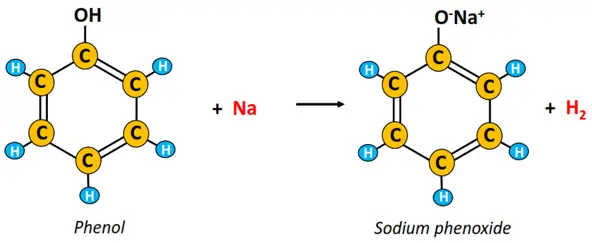
ii) Reaction with sodium metal
The chemical reaction of phenol with Na metal gives sodium phenoxide and hydrogen gas. The H2 gas spills out of the reaction mixture with a fizz.
iii) Electrophilic aromatic substitution reactions
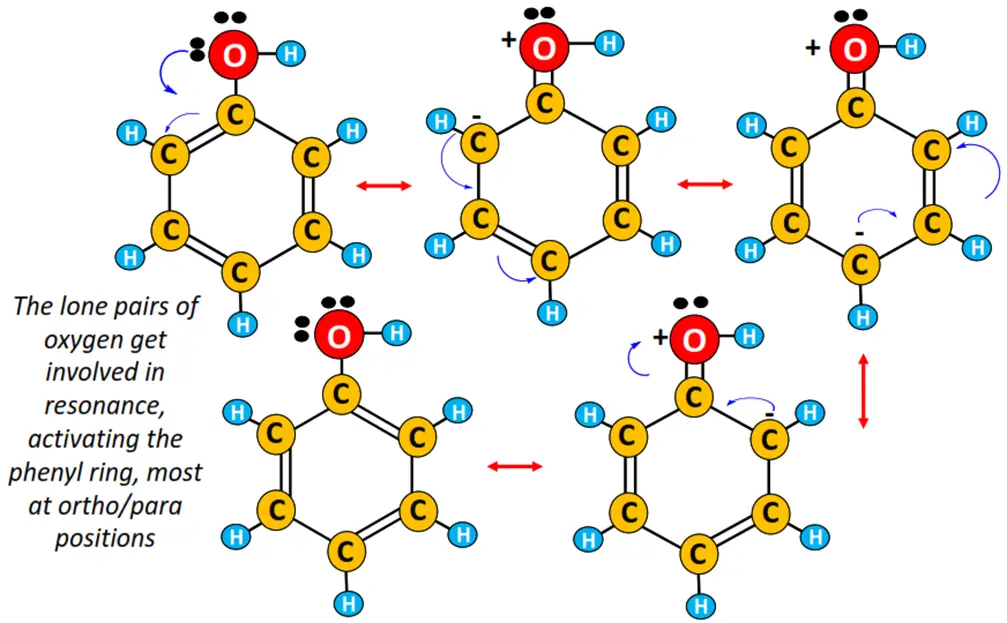
Phenols are substituted benzene molecules. So like parent benzene, it undergoes electrophilic substitution reactions. An OH group attached to benzene acts as the ring activator.
The lone pairs of electrons present on the O-atom get involved in resonance, thus, increasing the delocalized electron cloud within the phenyl ring, available for incoming electrophiles.
Also, an OH group attached to the benzene ring is ortho-para directing.
The nitration of phenol in the presence of conc. HNO3 and conc. H2SO4 gives o-nitrophenol as the major product. p-nitrophenol is also obtained, but as 2 ortho positions compete against 1 para position, the ortho product is obtained in bulk during electrophilic substitution reactions of phenols.

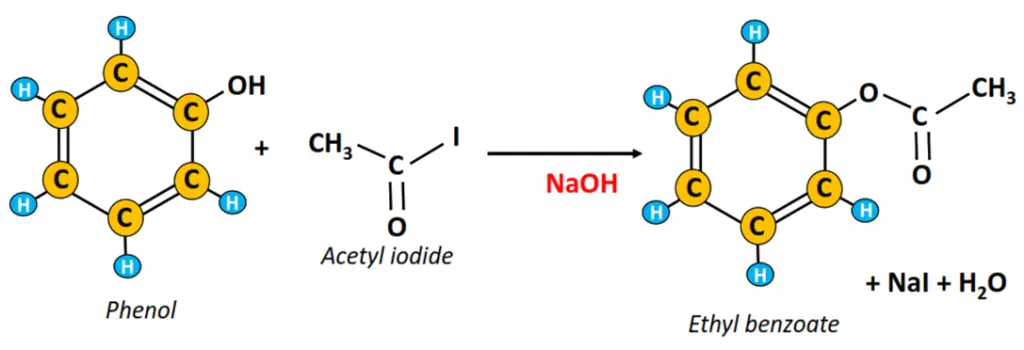
iv) Ester synthesis
The chemical reaction of phenol with an alkenyl halide (such as CH3COI) in the presence of a strong base (NaOH or KOH) gives an ester and the corresponding halide salt.
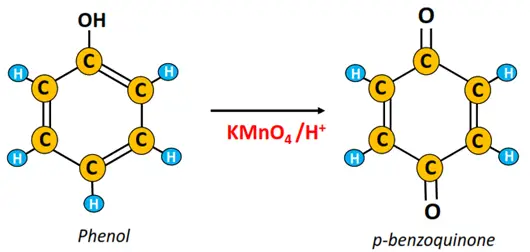
v) Oxidation reaction
The oxidation of phenol using acidified potassium permanganate (KMnO4) produces p-benzoquinone.
Why are phenols important? – Uses and Applications
- Phenols are used as raw materials in explosives, drugs and polymers, specifically Bakelite.
- It is used as an active pharmaceutical ingredient (API) in synthesizing lozenges and throat sprays due to its cooling effect.
- Phenol is used to sterilize medical equipment. It was the first organic compound to be used in antiseptic surgery back in 1865 by a British surgeon, Joseph Lister.
- Phenol is used as a preservative in vaccines designed against pneumonia, typhoid fever, smallpox, etc.
- Substituted phenols are used in the manufacturing of intensely colored azo dyes.
- Hydroxyphenol (hydroquinone) is used as a component in photographic film development.
- Methyl phenols (cresols) act as raw materials in wood preservatives.
- Complex phenols are also found naturally in plant-based essential oils used in flavorings and aroma therapy owing to their minty taste and odor. Some examples of naturally retrieved phenols or phenolic compounds are thymol and eugenol.
Which is more acidic; alcohols or phenols?
Phenols are more acidic than aliphatic alcohols.
Acids are proton donors. A phenol molecule liberates a proton (H+) ion to form the phenoxide ion.

The phenoxide ion is resonance stabilized and thus very stable. The different resonance structures of phenol and phenoxide ion are displayed below. Resonance neutralizes the negative charge present on the phenoxide ion.
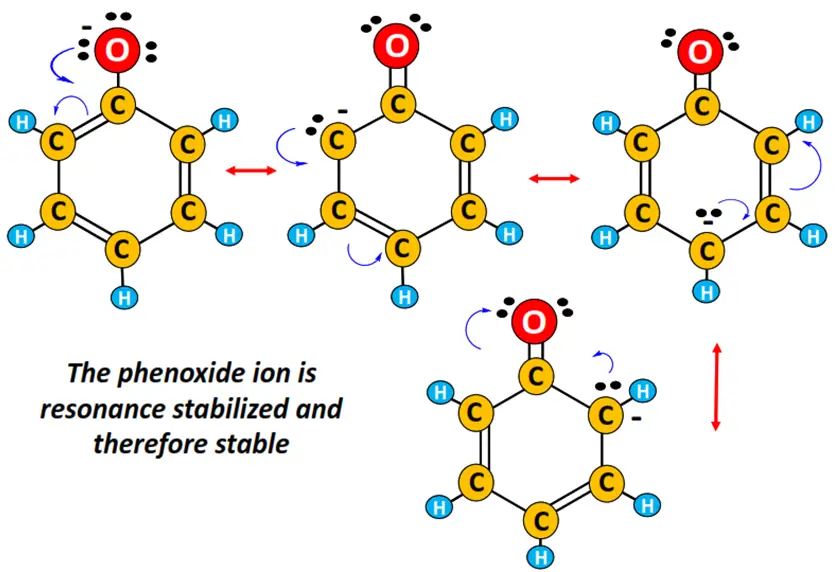
In contrast, the alkoxide ion produced by the loss of a proton by aliphatic alcohol is not resonance stabilized. It is rather unstable due to the O-atom’s highly concentrated negative charge. The alkyl chain attached to the O-atom is electron-donating, further increasing the negative charge on the O-atom, thus making it more unstable. The alkoxide ion combines with H+, and the ionization equilibrium shifts backward.

What is the chemical test to distinguish between alcohols and phenols?
The following chemical tests can be performed to distinguish between alcohols and phenols.
| Chemical Test | Observation with alcohols | Observation with phenols | Explanation |
| Ferric chloride (FeCl3) test. A solution of FeCl3 is added to the unknown sample | No color change is observed | A deep violet color is produced | A colored complex is formed between the ferric ions and the aromatic ring. |
| Bromine water test. Aq. bromine (Br2) solution is added to the sample | No color change | The red-brown color of bromine disappears | Phenol undergoes an electrophilic substitution (halogenation) reaction with bromine. |

Image by Tyler Johns, YouTube.

Moreover, phenols and aliphatic alcohols can also be distinguished from each other via spectroscopic analysis.
In the IR spectrum, the C-O stretching frequency is lower in phenols as opposed to aliphatic alcohols, as the C-O bonded electrons get involved in resonance within the ring. Conversely, different H-NMR and 13C-NMR peaks are obtained for the two different types of molecules.
You may also like introduction to organic chemistry.
References
1. Abdollahi, M., Hassani, S. & Derakhshani, M. 2014. Phenol. In: Wexler, P. (ed.) Encyclopedia of toxicology (third edition). Oxford: Academic press.
2. Morrison, R. T., Boyd, R. N. & Bhattacharjee, S. K. 2013. Organic chemistry.
3. Ouellette, R. J. & Rawn, J. D. 2015. 8 – Alcohols and phenols. In: Ouellette, R. J. & Rawn, J D. (eds.) Principles of organic chemistry. Boston: Elsevier.
4. Rappoport, Z. 2003. The chemistry of phenols., John Wiley & Sons, Ltd.
5. Sparkman, O. D., Penton, Z. E. & Kitson, F. G. 2011. Chapter 29 – Phenols. In: Sparkman, O. D., Penton, Z. E. & Kitson, F. G. (eds.) Gas chromatography and mass spectrometry (second edition). Amsterdam: Academic press.
