Benzene is a versatile chemical compound that has fascinated chemists for ages. It is greatly valued for its industrial and pharmaceutical applications. Benzene is widely used as a solvent, a precursor in the polymer industry, raw material for drug synthesis, etc.
But, more than its uses and applications, benzene attracts the scientific world’s attention due to its unique structure and exceptional stability.
Therefore, in this article, we will unlock the chemistry of benzene (C6H6).
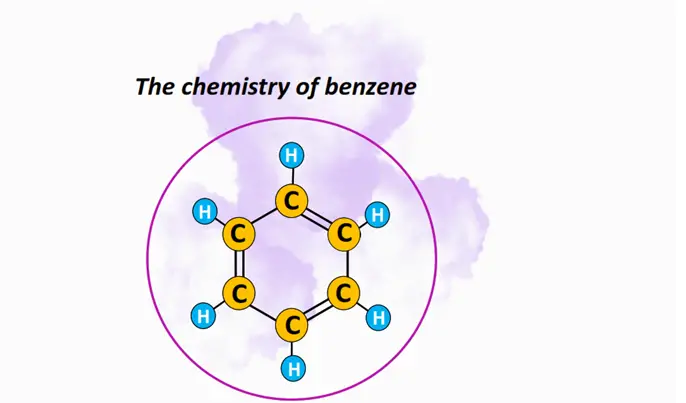
What is benzene? – Definition & Structure
Benzene (C6H6) is an aromatic hydrocarbon. Each benzene molecule comprises six carbon (C) atoms and six hydrogen (H) atoms. The six C-atoms are hexagonally arranged in a planar, ringed structure. The hexagonal ring arrangement consists of 3 C-C single and 3 C=C double covalent bonds at alternating positions. Thus, benzene is a conjugated molecule. It is a resonance stabilized, [6]-annulene, perfectly following the Huckle’s (4n + 2) π electrons rule.
Properties of benzene
- The molecular formula of benzene is C6H6. Its empirical formula is CH.
- Benzene is lighter than water (0.87 g/cm3).
- It exists as a clear, colorless, highly flammable liquid at r.t.p.
- The melting point of benzene is 5.5° C while its boiling point is 80.5°C.
- Benzene has a sweet, gasoline-like smell.
- Benzene is a non-polar solvent, freely miscible with other organic solvents but insoluble in water.
- It is a recognized carcinogen and is extremely toxic to human reproductive organs.
The discovery of benzene
Benzene was discovered in 1825 by an English chemist, Michael Faraday, who obtained benzene as a by-product of oil gas production. The basic structure of benzene was proposed by a German chemist, August Kekule, in 1865. However, it was not until 1931 that Linus Pauling, an American chemist, introduced the complex structure of benzene that we are familiar with today.
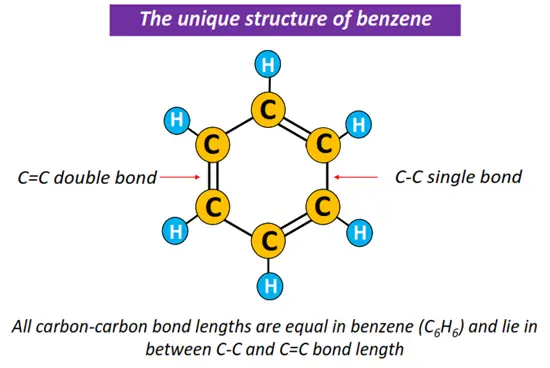
The basic benzene structure displays 3 C-C single bonds and 3 C=C double covalent bonds. A C=C double covalent bond tends to be shorter in length and thus stronger than a C-C single bond.
Contrarily, elemental analysis and X-ray diffraction studies performed on the benzene ring revealed that all carbon-carbon bond lengths are equal (1.39 Å) in a benzene molecule. It is intermediate between a C-C single bond length and double bond length, 1.54 Å and 1.33 Å, respectively. From here, scientists inferred that the structure of benzene is a bit more than what appears superficially.
The benzene molecule is resonance stabilized. Let’s find out more about that.
Electron delocalization and resonance in benzene
Benzene is a symmetrical, planar molecule. All 6 C-atoms lie flat in the hexagonally arranged benzene ring, making an H-C-C bond angle of 120°. Each carbon atom is sp2 hybridized in benzene, implying that all six C-atoms possess an unhybridized p-orbital, as well.
A C-atom uses its unhybridized p-orbital to form a pi bond with the adjacent C-atom by parallel p-p orbital overlap.
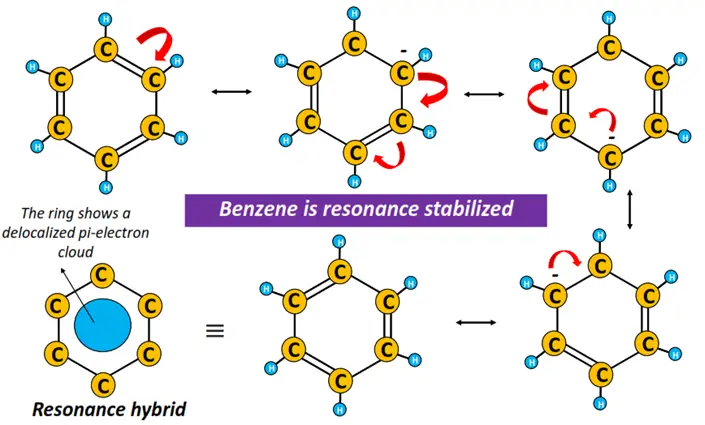
An interesting point here is that, after pi-bond formation, the constituent atomic orbitals combine to form molecular orbitals. As a result, the electrons present in the π molecular orbitals do not stay confined between two atomic nuclei; rather, they are delocalized and belong to the benzene molecule as a whole.
The presence of alternating single and double bonds between adjacent C-atoms in the benzene ring is known as conjugation.
In contrast, the continuous movement of the pi-bonded electrons from one position to another on the molecule is referred to as resonance.
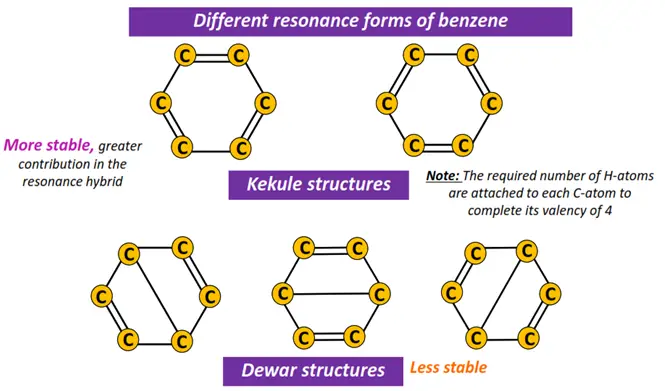
Benzene can be represented by the following different types of Lewis structures, depending upon the possible location of the double bonds. These include 2 Kekule structures and 3 Dewar structures.
The actual structure of benzene is a hybrid of all the possible canonical forms; hence it is known as the resonance hybrid.
The Kekule structures contribute more (80 %) to the resonance hybrid as compared to the Dewar structures (20% contribution).
Thus, the resonance hybrid looks more like the Kekule representation. The delocalized pi-electron cloud is shown as a ring circulating freely within the hexagon in the resonance hybrid.
Stability of benzene
Unlike cyclohexene, benzene does not decolorize bromine (Br2) water. This is because Br2 cannot break C=C double bonds in benzene under normal temperature conditions; thus, no addition reaction occurs.
This extra stability of benzene is accredited to its resonance stabilization. The delocalized pi-electron cloud gets preoccupied with resonance within the benzene ring. Therefore, external chemical reagents cannot easily approach it.
Rather, benzene undergoes electrophilic substitution reactions under special conditions in which the resonance-stabilized ring system is preserved.
Moreover, it is due to the exceptional stability of benzene that its heats of hydrogenation and combustion are lower than that expected. In contrast, the resonance energy of benzene (the energy difference between the real molecule and a hypothetical Kekule structure) is 36 kcal/mol.
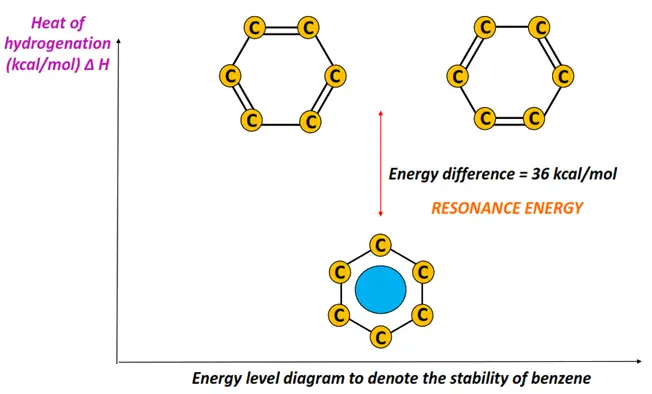
Important chemical reactions of benzene
Benzene undergoes the following electrophilic substitution reactions:
i) Nitration
The chemical reaction of benzene with conc. nitric acid (HNO3) in the presence of conc. sulfuric acid (H2SO4) gives nitrobenzene.
The nitronium (NO2+) ion produced in-situ acts as the electrophile.
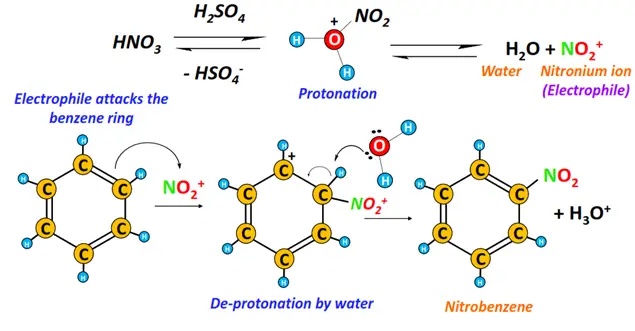
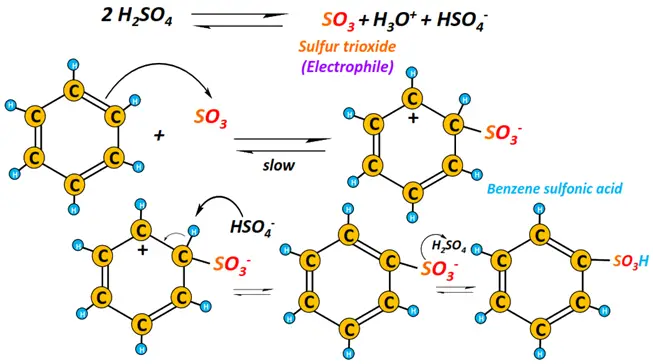
ii) Sulfonation
Heating benzene with fuming sulfuric acid (HOSO3H) produces benzene sulfonic acid. Sulfur trioxide (SO3) gas is the electrophile that attacks the benzene ring in this case.
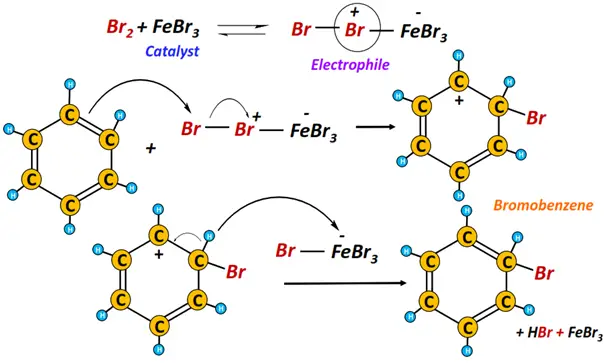
iii) Halogenation
The halogenation of benzene (with Cl2 or Br2) using an iron catalyst yields an aryl halide, releasing hydrogen halide gas as a byproduct.
The halonium (X+) ion acts as an electrophile.
iv) Friedal Craft alkylation
The chemical reaction of benzene with an alkyl halide in the presence of a Lewis acid (such as AlCl3) produces alkyl benzene, while hydrogen chloride is released as a byproduct.
A carbocation functions as an electrophile.
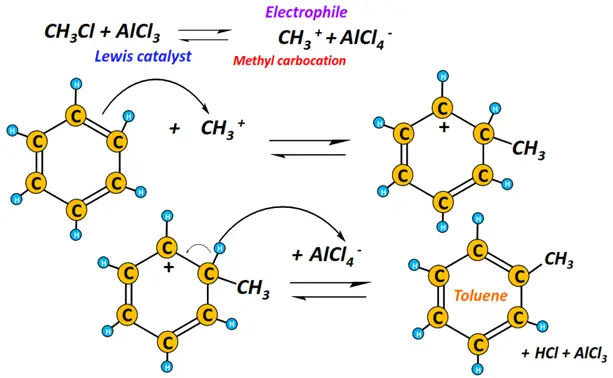
v) Friedal Craft acylation
The chemical reaction of benzene with an acyl chloride in the presence of AlCl3 gives a ketone of corresponding chain length, while hydrogen chloride gas is produced as a by-product.
The acylium ion functions as the electrophile.
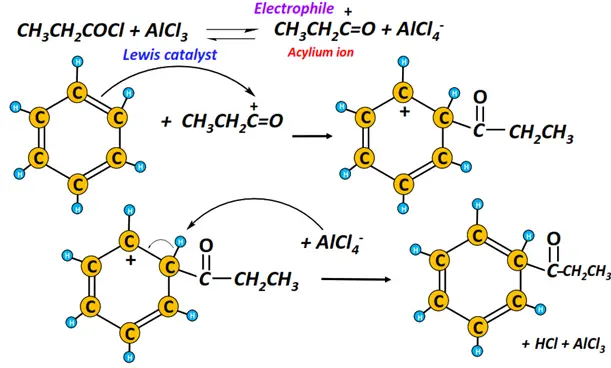
vi) Diazo coupling
An azo compound (organic dye) is formed by the coupling reaction between benzene and diazonium salt.
In this case, the diazonium ion acts as an electrophile.

A series of benzene derivatives are produced by the different types of chemical reactions that a benzene molecule undergoes.
A particular functional group attached to the benzene ring directly affects its reactivity and orientation for further substitutions.
A monosubstituted benzene ring thus reacts differently from the parent benzene molecule. A functional group attached to benzene can be an activating group or a deactivating group. Similarly, it can either be an ortho-para directing group or a meta-director. All ring deactivators are meta directors, except halogens.
Below are examples of prominent ring activators, deactivators, ortho-para and meta directors controlling the chemistry of benzene.
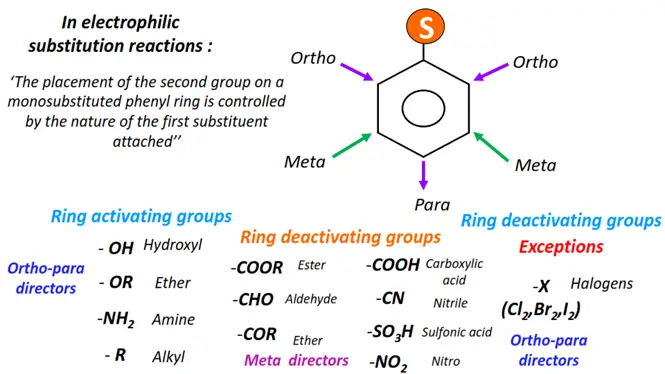
Find more about different organic chemistry reactions and mechanisms in our subsequent articles.
Why is benzene important? – Uses & Applications
- Benzene is a versatile solvent. In the chemistry laboratory, it dissolves fats, oils and waxes.
- Benzene is used as a precursor in the polymer industry for manufacturing rubber and plastics (polyester, polystyrene, nylon, etc.)
- Benzene derivatives such as phenol and aniline are used to produce detergents and pharmaceutical drugs.
You may practice this worksheet to revise your concepts on the chemistry of benzene.
References
1. H.Brown, W. & Poon, T. 2016. Introduction to organic chemistry.
2. M.Younas 2015. A textbook of organic chemistry.
3. Morrison, R. T., Boyd, R. N. & Bhattacharjee, S. K. 2013. Organic chemistry.