The term isomerism unlocks a fascinating world in organic chemistry. It deals with how a multitude of distinct atomic arrangements can be hidden in a single molecular formula, generating endless possibilities for synthesizing diverse organic compounds.
The word isomer combines two Greek words, i.e., isos (meaning plus) and meros (that denotes equal parts). Hence, isomers logically refer to those chemical compounds that possess equal parts but are still different.
Therefore, without any further delay, dive into the article and learn all there is to know about isomerism in organic chemistry.
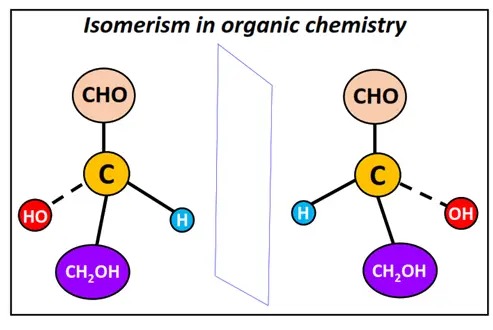
What is isomerism -Definition and Examples
Isomers are defined as chemical compounds possessing the same molecular formula but a different structural arrangement. The structural arrangement and connectivity of constituent atoms may be deciphered in two dimensions or three dimensions. This leads to the diverse physical and chemical properties of different isomeric compounds.
Isomerism is a combined study of the different types of isomers, their structures and properties.
Examples:
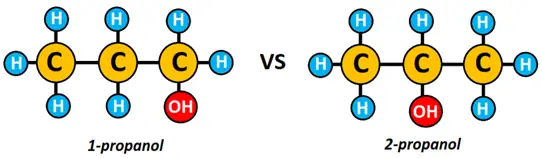
- 1-propanol and 2-propanol are isomers of each other, differing in the placement of the hydroxyl (OH) functional group. However, both are represented by the same chemical formula, i.e., C3H8O.
- Ethanol and dimethyl ether are isomers of each other. Both are represented by the same molecular formula, i.e., C2H6O; however, the type of functional group present in the two molecules differ. Ethanol is an alcohol, while dimethyl ether belongs to the ether family.
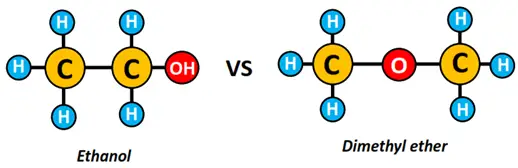
Now let’s see in detail how we have differentiated between the two different types of isomers above.
Different types of isomerism in organic compounds
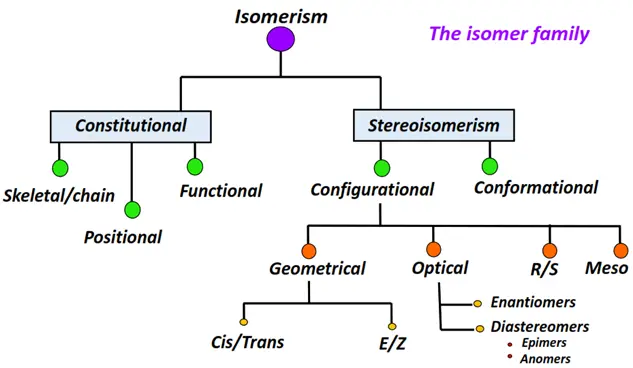
The two main types of isomerism in chemistry are:
- Constitutional isomerism
- Stereoisomerism
Constitutional isomerism
Constitutional isomers are organic compounds with the same molecular formula, but the sequence in which the constituent atoms are bonded differs. Such types of isomers can be distinguished from one another based on their two-dimensional structures. Thus, constitutional isomers are also sometimes known as structural isomers.
Constitutional/ structural isomers are sub-divided into:
o Skeletal or chain isomerism
Organic compounds can form straight chain structures as well as branched molecules.
Two compounds with the same molecular formula but different bond connectivity are called chain isomers.
For example, n-butane and isobutane are chain isomers represented by C4H10. n-butane is a straight chain structure, while in isobutane, a methyl (CH3) group is attached to C-2 forming a branched molecule, as shown below.
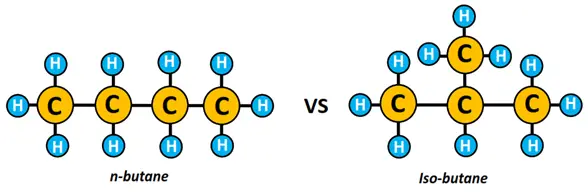
o Positional isomerism
Positional isomers are formed if the position of a functional group differs between two related molecules.
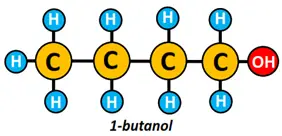
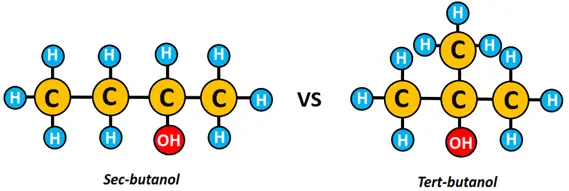
For instance, 1-butanol, sec-butanol, and tert-butanol are positional isomers.
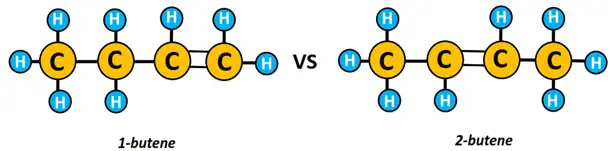
Another example of positional isomers is 1-butene and 2-butene, differing in the position of the C=C double covalent bond.
Similarly, the alcohol molecules, 1-propanol and 2-propanol, we encountered in the previous section are positional isomers of each other.
Mind Buzz: Are 1-butanol and iso-butanol also positional isomers?
Answer: Apparently, they look like positional isomers, but if you notice closely, the placement of the OH group is at C-1 in both cases. However, 1-butanol is a straight-chain molecule, while iso-butanol is a branched structure.
Thus, we can safely say that 1-butanol and iso-butanol are chain isomers.

Now let us move on to the third type of constitutional isomerism.
o Functional isomerism
Functional isomers are constitutional isomers possessing the same molecular formula but a different functional group moiety.
For example:
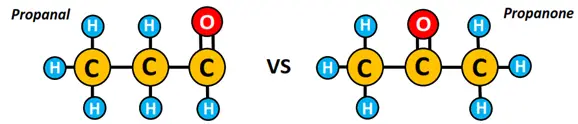
The formula C3H6O can represent two different molecules, i.e., propanal and propanone. Propanal and propanone are functional isomers as one is an aldehyde (represented by the CHO group) while the other is a ketone (possessing an R-C=O-R group).
Stereoisomerism
Stereoisomers are chemical compounds possessing the same molecular formula as well as the same bond connectivity in 2-D. However, their three-dimensional arrangement in space differs.
Stereoisomerism is further divided into two main types:
o Configurational isomerism
Configurational isomers are stereoisomers that cannot be interconverted by simply rotating a sigma bond.
These are sub-categorized into:
- Geometrical isomers
- Optical isomers
o Conformational isomerism
Conformational isomers are stereoisomers that are interconvertible by simply rotating one part of the molecule with respect to the other by a single/sigma bond.
It does not involve any bond breaking or making. However, certain energy changes do take place. Conformational isomers are also known as conformers or rotamers.
Example:
4 different conformational isomers of cyclohexane (C6H12)
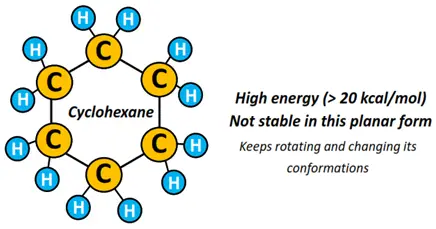
The molecular geometry of cyclohexane w.r.t each C-atom is tetrahedral. In contrast, it possesses C-C-C bond angles equal to 120° in the regular hexagon.
Therefore, the hexagonal ring structure keeps rotating at room temperature, adopting different conformations to bring the bond angles closer to an ideal value of 109.5° (tetrahedral angle), reducing overall ring strain.
The four cyclohexane conformations include:
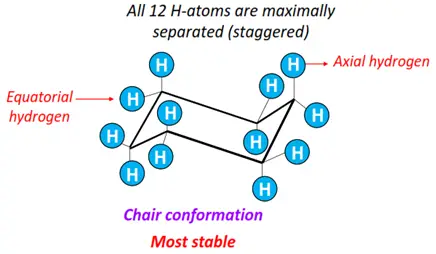
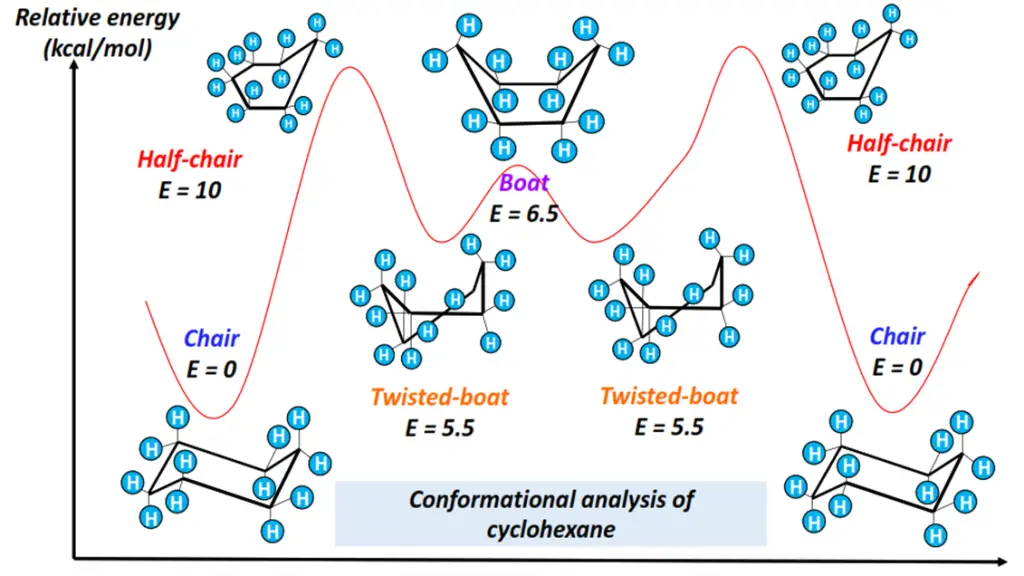
- Chair form: All 12 H-atoms are staggered. The bond angles are close to the ideal value of 109.5°. It is thus the most stable conformation of cyclohexane, possessing the lowest energy as it is free of all ring and torsional strains.
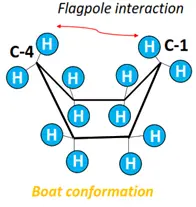
- Boat form: Flagpole interaction exists between the H-atoms attached at C-1 and C-4, decreasing the molecule’s stability.
- Twist boat form: Less stable than the chair form but more stable than the boat conformation of cyclohexane. Twist boat form is flexible as it undergoes a slight twist, reducing torsional strain and flagpole interactions.
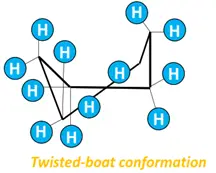
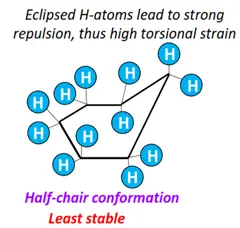
- Half-chair form: The least stable conformational isomer of cyclohexane, possessing maximum ring strain and energy. The H-atoms are eclipsed by one another.
Specific energy changes occur as the cyclohexane molecule rotates, adopting different conformations, as shown in the energy profile diagram drawn below.
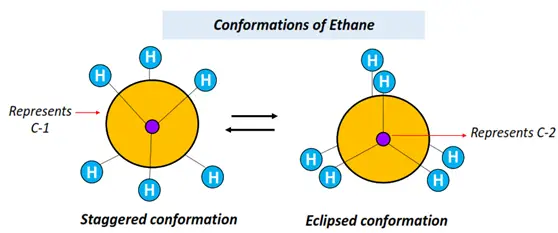
Conformational isomers of acyclic alkanes, such as ethane, propane, butane, etc., can be shown using the Newman projection.
Refer to the figures drawn below.
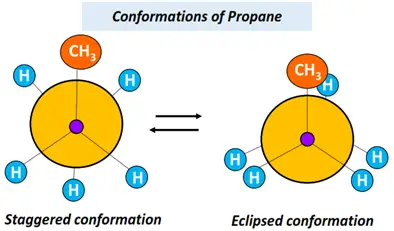
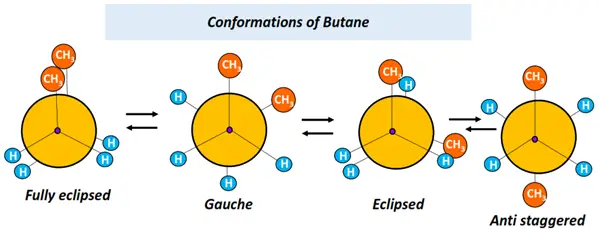
Now let us go back to the two different types of configurational isomers.
Geometrical isomerism
Geometrical isomers are formed by bond breaking or new bond formation between two carbon atoms in an organic molecule. It is predominantly studied in unsaturated hydrocarbons, i.e., alkene molecules possessing one or more C=C double covalent bonds.
A C=C double bond allows restricted rotation; therefore, different geometrical isomers are produced as the C=C bond breaks, changing its position.
IUPAC (The International Union of Pure and Applied Chemistry) now prefers using the terms cis/trans isomerism and E/Z isomerism for geometrical isomers.
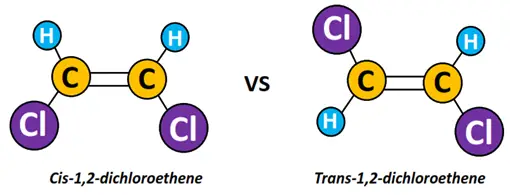
Cis-trans isomerism
Cis-trans isomerism exists in alkene molecules in which the same groups are attached on two sides of the C=C double bond.
For example, In 1,2-dichloroethene, two Cl-atoms are attached on double covalently bonded adjacent C-atoms. In cis-dichloroethene, both the Cl-atoms are attached on the same side. Contrarily, in trans-dichloroethene, the Cl-atoms are attached on opposite sides of the molecule, as shown below.
E/Z isomerism
Alkene molecules can still exhibit geometrical isomerism even if the C=C atoms do not possess common functional group moieties. In this case, it is known as E/Z isomerism. The letters E and Z represent the Greek words Entgegen and Zusammen, respectively.
E-Z configuration is an extension of the cis-trans notation that can be used to describe alkene molecules having two, three or four different substituents.
For instance, cis-trans notation is not applicable to the molecules shown below. But we can assign these an E/Z configuration based on the following rules:
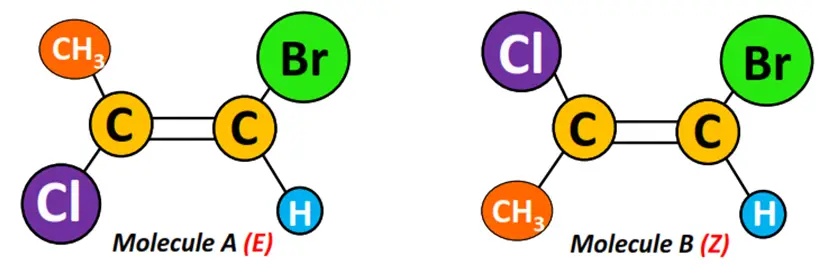
- Z-configuration: High-priority groups are present on the same sides of the double bond.
- E-configuration: High or Low priority groups are located on the opposite sides of the double bond.
- A high-priority group is one that has a higher molecular weight between the two groups attached to a C-atom.
- If the bonded groups are isotopes of the same element, the atoms of higher mass numbers have the higher priority.
In the molecules shown above, Cl (mass number = 35.5) is a high-priority group on C-1 as compared to CH3 (molar mass = 15). In contrast, Br (mass number = 80) is a high-priority group as opposed to H (mass number = 1) on C-2.
Therefore, A and B are geometrical isomers of each other. Molecule A is in E- configuration, while Molecule B is in Z-configuration.
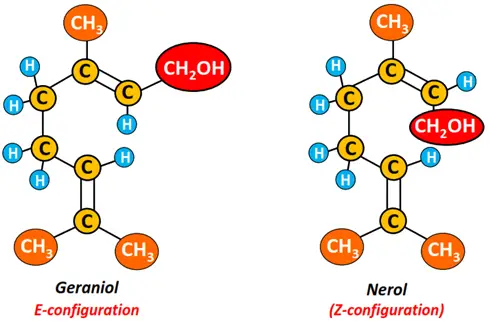
Conversely, geraniol and nerol are organic compounds in the rose flower, giving it its characteristic fragrance. Both are geometrical isomers; geraniol is the E-isomer, while nerol has a Z-configuration.
Another less commonly discussed type of stereoisomerism is R/S isomerism.
R/S isomerism
It is a type of isomerism present in saturated hydrocarbons that possess a chiral center.
A chiral center is an atom in an organic molecule tetrahedrally bonded to four different chemical species.
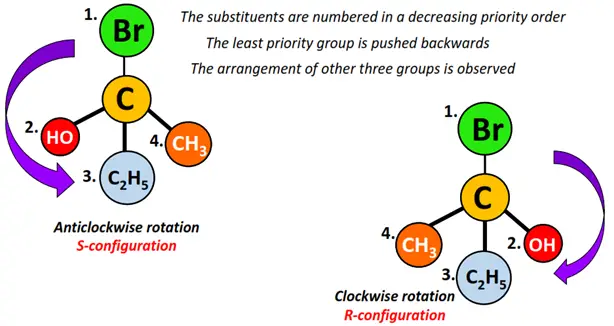
R/S notation is given to molecules following the CIP sequence rules.
After assigning a priority sequence (similar to E/Z configuration) to the atoms attached to the chiral center, the molecule is visualized in 3-D such that the group of lowest priority is directed away from the observer.
In assigning the other groups from that of higher priority to second and third, if the viewer’s eye travels in a clockwise direction, the molecule is assigned R-configuration (R for the Latin word rectus meaning right). Contrarily, if the eye movement follows a counterclockwise rotation, the molecule is assigned an S-configuration (S for sinister, meaning left).
Optical isomerism
Optical isomers are chemical compounds that rotate the plane of vibration of monochromatic, plane-polarized light in opposite directions but to the same extent.
Light refers to the visible region (400-800 nm) of the electromagnetic spectrum. Unpolarized electromagnetic radiations travel in different directions. If a specific wavelength is selected from a range of light wavelengths, it is known as monochromatic light. The monochromatic light, when passed through a polarizer, is made to travel in a specific direction only. It is thus referred to as plane-polarized light.
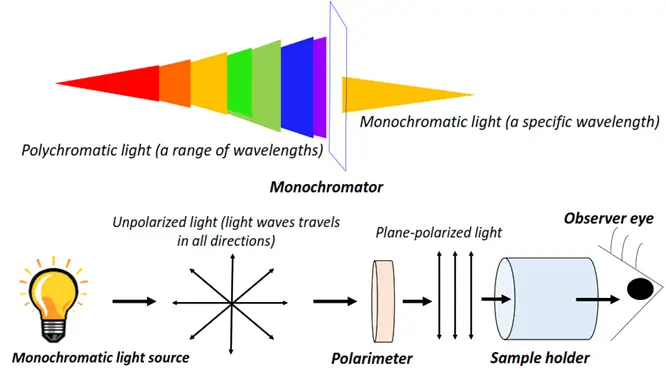
Optical activity denotes the ability of an organic molecule to rotate or change the plane of vibration of the plane-polarized light as it passes through the sample.
Optical isomers are asymmetric molecules, often possessing a chiral center. However, not all chiral substances are optically active in nature.
Optical isomers are also known as enantiomers as they form non-super impossible mirror images of each other.
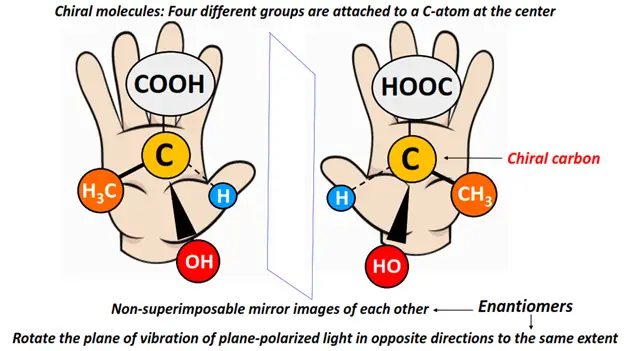
The enantiomer that rotates the plane of vibration of plane-polarized light clockwise is called dextrorotatory (d or +). In contrast, that which rotates it anticlockwise is known as a levorotatory (l or -) isomer.
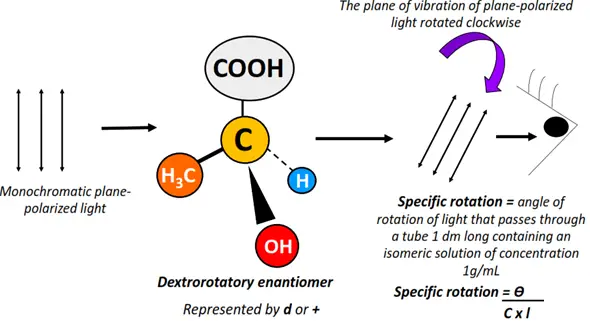
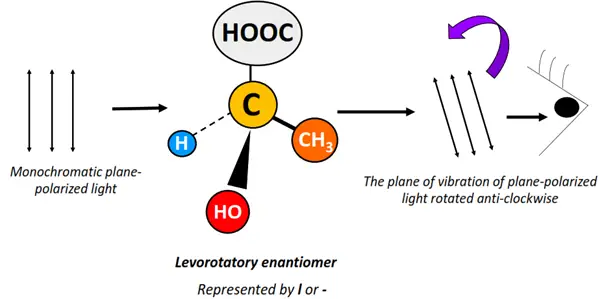
Enantiomers possess the same molecular formula, structural arrangement and even physical properties. However, they differ only in their behavior towards plane-polarized light, leading to a difference in their distinct chemical properties.
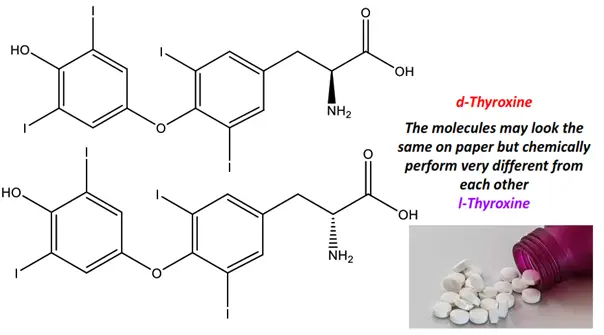
For instance, l-thyroxine is the l-enantiomer of thyroxine, an important medicinal drug used to treat hypothyroidism, while d-thyroxine has a negligible hormonal effect on human body tissues.
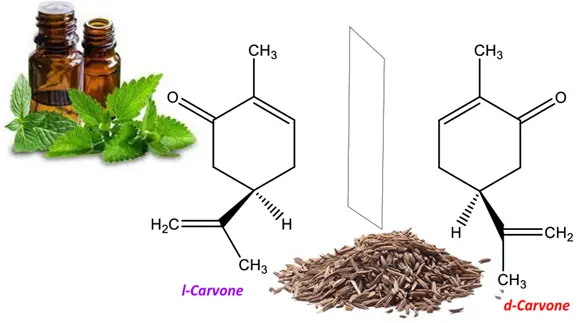
In another example, l-carvone is a levorotatory enantiomer that gives the oil of spearmint its characteristic odor. Contrarily, the dextrorotatory enantiomer d-carvone is the essence of caraway.
Racemic modification and resolution
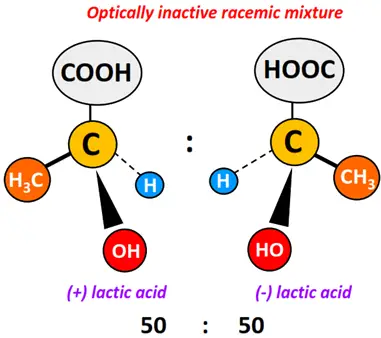
When two enantiomers are present in an equal proportion (50:50) in a sample mixture, it is known as a racemic modification or racemate.
The plane-polarized light entering a racemic mixture does not undergo any rotation as the optical activity of the two enantiomers gets canceled completely.
The prefix ± is used to represent a racemic modification. For example, ± lactic acid.
Lactic acid is an alpha-hydroxy acid, as we saw in the previous section.
The IUPAC name for lactic acid is 2-hydroxy propanoic acid. Its structure is as shown below.
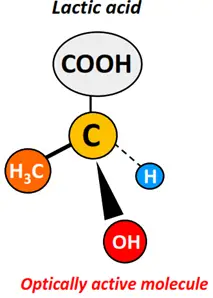
It is an asymmetric molecule possessing a chiral carbon that is single-covalently bonded to four different groups at the sides. These include an H-atom, a CH3 group, an OH group and a carboxylic acid (COOH) functional group. Lactic acid is thus an optically active molecule.
But when an equal proportion of dextrorotatory and levorotatory lactic acid molecules are mixed, the optical rotation of one isomer is canceled by an equal but opposite rotation induced by the other isomer. Therefore, the mixture itself is optically inactive.
However, a few techniques exist to separate the constituent enantiomers present in a racemic modification. This process is called the resolution of the racemic modification.
Methods for the resolution of a racemic modification
- Mechanical method: Seeding the racemic mixture with a substance that only crystallizes one of the two enantiomers, followed by hand-picking easily recognizable crystals using a lens and tweezers. However, this method is seldom used and rarely useful.
- Biochemical method: Treating the racemate with an enzyme, which recognizes one enantiomer only and converts it into an easily isolable product.
- Kinetic method: Reacting the racemate with a chiral catalyst. The two enantiomers react at different rates, resulting in the enrichment of the less reactive enantiomer.
- Chemical method: Chemically reacting the racemic mixture with another optically active reagent. It converts the racemic mixture into a mixture of diastereomers which can easily be separated.
Diastereomers
Diastereomers are optically active stereoisomers that possess the same molecular formula, similar bond connectivity and are also non-superimposable like enantiomers. However, they differ from enantiomers based on the fact that diastereomers are not mirror images of each other.
The concept of diastereomerism is most relevant in sugar molecules.
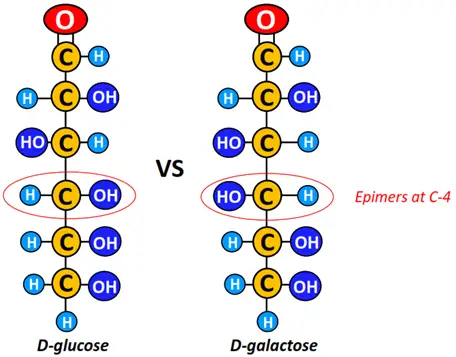
For example, D-glucose and D-galactose are diastereomers; their structures are as shown below.
Epimer is one of a pair of diastereomers. Thus, D-glucose is epimeric to D-galactose at C-4. Epimers differ in their configuration at only 1 chiral center. All epimers are diastereomers, but the opposite may not be true.
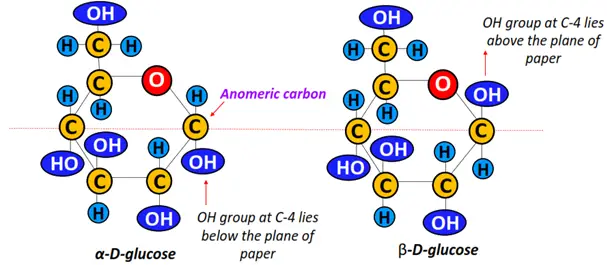
Furthermore, anomers are a sub-type of epimers that form cyclic structures. An example includes alpha- D-glucose and beta-D-glucose. The structure of the two molecules differs at the anomeric carbon, i.e., C-4 in this case.
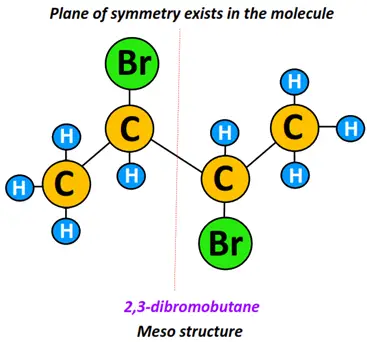
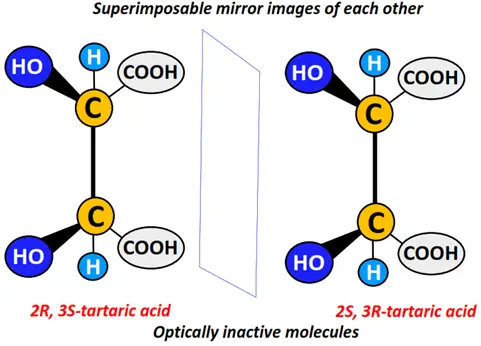
Meso structures
In contrast to diastereomers, meso structures are symmetrical stereoisomers that form superimposable mirror images of each other. Meso structures are thus optically inactive, although they may have two or more chiral centers.
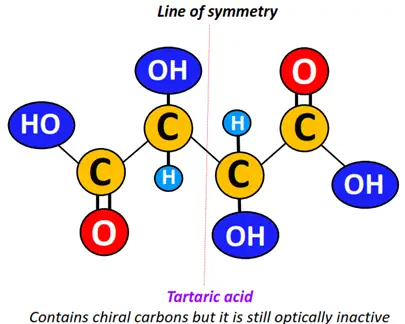
Examples:
2,3-dibromo butane is a meso structure. It possesses a stereogenic, chiral center. However, a plane of symmetry exists in the molecule, making its mirror image superimposable. Therefore, the molecules shown below fall under meso isomers.
Similarly, 2R, 3S-tartaric acid and 2S, 3R- tartaric acid are names used to represent the superimposable mirror images of tartaric acid, a meso compound.
Lastly, we would like to introduce you to two other important terms in organic isomerism, i.e., tautomerism and metamerism.
What is tautomerism in organic chemistry?
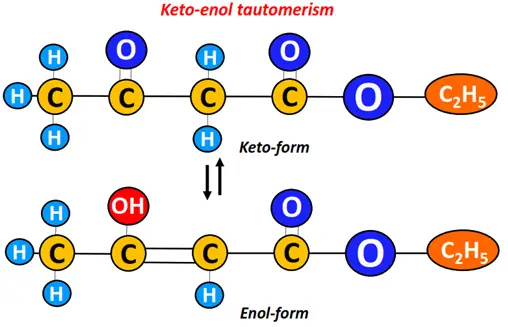
Tautomerism is a special kind of dynamic, structural isomerism that exists in organic molecules.
Tautomers are interconvertible structures formed as a result of the movement of a single atom from one position to another on the molecule, followed by a consequent relocation of a double bond.
For example, in keto-enol tautomerism, a double bond and the H-atom change their position, yielding two different structures, as shown below.
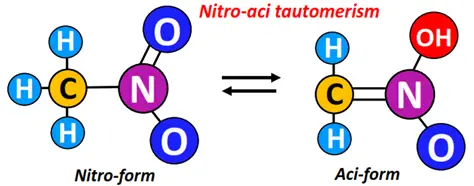
Conversely, in nitro-aci tautomerism, the position of the H-atom changes along with converting a nitro group to an aci form, as shown below.
What is metamerism in organic chemistry?
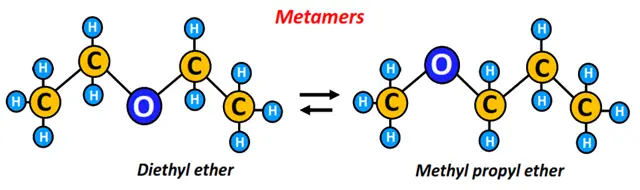
Metamers are constitutional isomers having different numbers of carbon atoms around a functional group, such as the ether (R-O-R) group or the amine (-NH-) group.
Example: Diethyl ether and methyl propyl ether are metamers. Both are represented by the same molecular formula, i.e., C4H10O; however, the length of the carbon chain on either side of the oxygen atoms differs in the two molecules, as shown below.
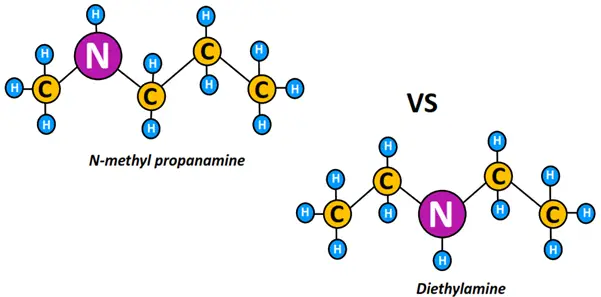
Another example of metamers is n-methyl propanamine and diethyl amine.
Check out this interesting source if you want to revise your concepts on isomerism.
Test your knowledge on isomerism here.
If you found this article helpful, we invite you to have a look at another interesting read on organic spectroscopy.
References
1.Ashenhurst, J. 2022. Types of isomers: constitutional isomers, stereoisomers, enantiomers, and diastereomers. Stereochemistry and chirality [online].
2. Jones, M. 2023. Isomerism In: Britannica, T. E. O. E. (ed.) Britannica.
3. M.Younas 2015. A textbook of organic chemistry.
4. Morrison, R. T., Boyd, R. N. & Bhattacharjee, S. K. 2013. Organic chemistry.
5. Wermuth, C. G. 2015. Chapter 18 – Optical isomerism in drugs. In: Wermuth, C. G., Aldous, D., Raboisson, P. & Rognan, D. (eds.) The practice of medicinal chemistry (fourth edition). San Diego: Academic Press.
