Aldehydes and ketones form an essential part of organic chemistry. The adorable vanilla fragrance of candles, perfumes, ice creams and freshly baked food comes from vanillin, an aldehyde. On the other hand, ketones are present in natural animal and plant-based compounds. The human body’s metabolism also produces ketones, from which comes the name of the ever-famous keto diet.
Both aldehydes and ketones have a carbonyl (C=O) group in common. It depends upon the placement of the carbonyl functional group, whether it is an aldehyde or a ketone.
In this article, we have described the chemistry behind aldehydes and ketones.
Continue reading to learn more and all you need to know about aldehydes and ketones.
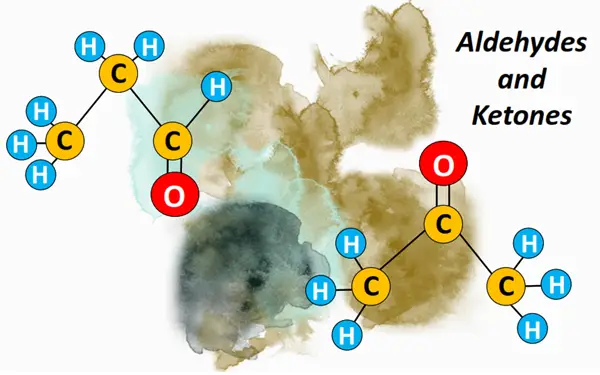
What is an aldehyde? –Definition & Structure
An aldehyde is a carbonyl compound containing a CHO functional group. The C=O bond is present at the terminal position, i.e., carbon no. 1. The CHO group may be bonded to an aromatic or aliphatic (acyclic) carbon chain. Aliphatic aldehydes are represented by a general formula CnH2n+1CHO.
Methanal, more commonly known as formaldehyde (HCHO), is the first member of the aldehyde family. It is followed by ethanal or acetaldehyde (CH3CHO), propanal (CH3CH2CHO), butanal (CH3CH2CH2CHO), etc.
What is a ketone? –Definition & Structure
A ketone is a carbonyl compound in which the carbon atom carrying the C=O group is covalently bonded to two other C-atoms. Aliphatic ketones are represented by a general formula R-CO-R. The two alkyl chains at the sides (R) may be equal or different in length.
Propanone, commonly known as acetone (CH3-CO-CH3), is the first member of the ketone family. It is followed by butanone (methyl ethyl ketone), 2-pentanone (methyl propyl ketone), and so on. The prefix 2 represents the C=O group located at carbon number 2 in the alkyl chain.
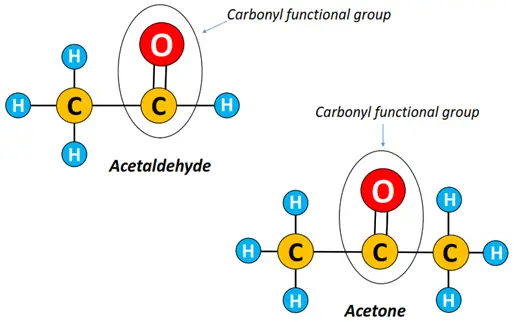
What is the difference between aldehydes and ketones?
As discussed above, both aldehydes and ketones are carbonyl compounds in which the C=O group largely determines their physicochemical properties.
However, some key differences present between the two organic compounds are as follows:
| Aldehydes | Ketones |
| C=O lies at the terminal position | C=O lies within the alkyl chain. |
| Represented by R-CHO | Represented by R-CO-R’. |
| Less polar than ketones | Generally more polar than aldehydes due to the presence of two electron-donating R groups at the sides of C=O. |
| Lower boiling points | Relatively higher boiling points, so greater stability. |
| More reactive than ketones | Comparatively less reactive than aldehydes. |
| Oxidizes to carboxylic acid | Cannot be further oxidized. |
| Reduces to a primary alcohol | Undergoes reduction to produce secondary alcohol. |
| Good reducing agents | Poor reducing agents. |
Tests to differentiate between aldehydes and ketones
An aldehyde can be distinguished from a ketone-based on the following chemical tests:
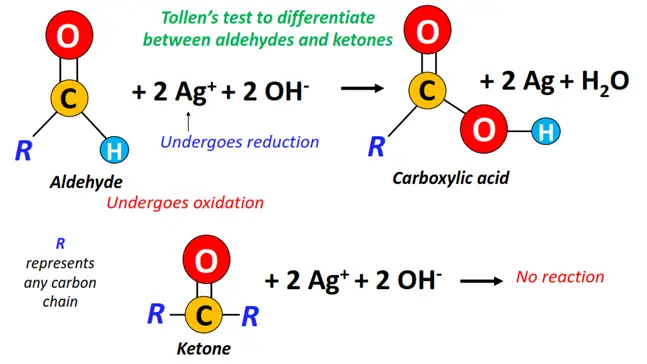
Silver mirror test
Tollen’s reagent is an alkaline solution of ammoniacal silver nitrate (Ag(NH3)2+). If an aldehyde is heated with Tollen’s reagent, the aldehyde gets oxidized to a carboxylate (COO–) anion (under alkaline conditions) while reducing Ag+ ions to silver (Ag) metal. Metallic silver gets deposited on the walls of the test tube and forms a silver mirror.
However, no such reaction occurs when reacting a ketone with Tollen’s reagent. Thus, no silver mirror formation in the presence of a ketone.

Image by sciencephoto.com
Fehling’s solution test
Fehling’s reagent refers to an alkaline solution of copper sulfate. It is blue in color. On heating a small amount of Fehling’s solution with an aldehyde, the cupric (Cu2+) ions are reduced to cuprous (Cu+) while the aldehyde oxidizes to a carboxylate ion.
A brick-red cuprous oxide (Cu2O) precipitate is formed, denoting an aldehyde’s presence in the sample mixture.
However, no brick-red ppt. is formed with a ketone. Thus, it helps differentiate between an aldehyde and a ketone.
Aldehydes and ketones- Nomenclature & Examples
The generic name for an aldehyde is alkanal.
The alphabet ‘e’’ from the name of an alkane is removed and replaced by the suffix ‘al’’ to represent the aldehyde of the corresponding chain length.
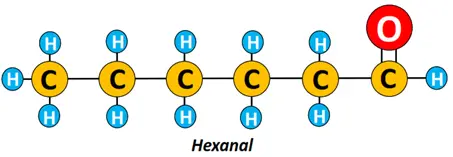
Example: For an aldehyde molecule containing 6 carbon atoms, the letter ‘e” is removed from hexane, and ‘al” is added. Thus, its name is hexanal.
Conversely, the generic name for a ketone is alkanone.
In this case, the alphabet ‘e’’ of an alkane is removed and replaced with the suffix ‘none’’.
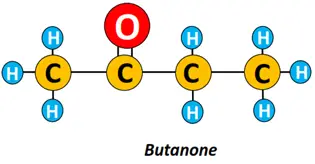
Examples: Butanone is a 4-carbon ketone derived from butane.
There are 2 possible isomers for a ketone containing 5-carbon atoms, i.e., Pentanone. The carbonyl (C=O) functional group can be present at C-2 or C-3, forming 2-pentanone or pentan-2-one and 3-pentanone, respectively.
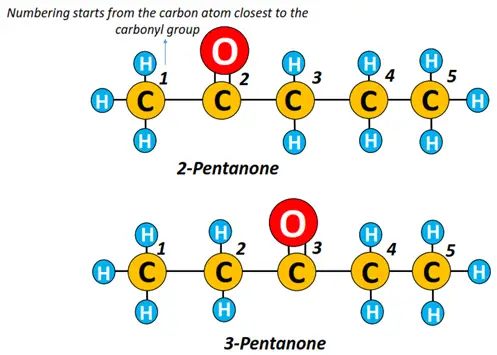
Pro-tip: Always remember to number the long alkyl chain, starting from the carbon that lies nearest to the carbonyl (C=O) functional group for all types of aldehydes and ketones.
Preparation and reactions of aldehydes and ketones
Aldehydes and ketones undergo addition reactions, including both nucleophilic as well as electrophilic addition. The C=O bonded carbon atom acts as an electrophilic (electron-deficient) site. Contrarily, the C=O bonded oxygen atom acts as a nucleophilic (electron-rich) reactive center in aldehydes and ketones. Most reactions occur at the electrophilic site.
Aldehydes and ketones can be prepared by:
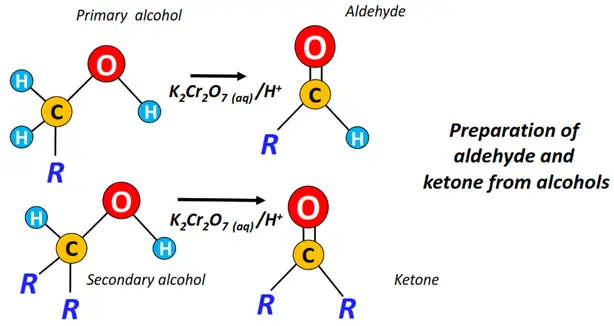
- The controlled oxidation of primary alcohols (for aldehydes) and complete oxidation of secondary alcohols (for ketones).
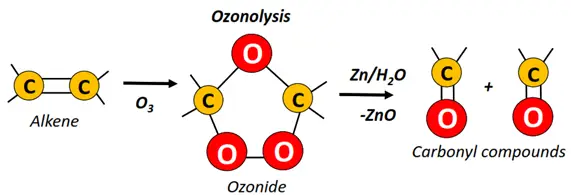
- Ozonolysis of alkenes.
- Friedel-Crafts acylation of Aromatics.
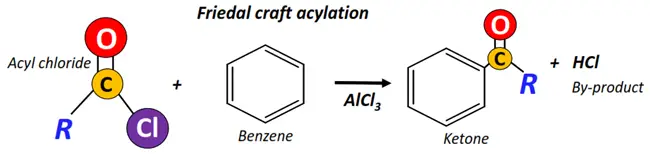
The most important chemical reactions of aldehydes and ketones are:
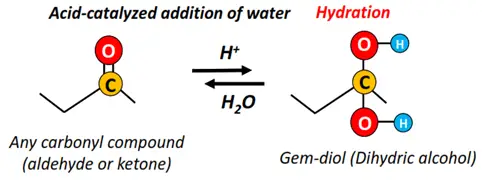
- Nucleophilic addition of water
A water (H2O) molecule is added across the C=O double bond to yield the corresponding gem-diols. The C=O double bond breaks and 2 OH groups are added onto this carbon atom to form a dihydric alcohol.
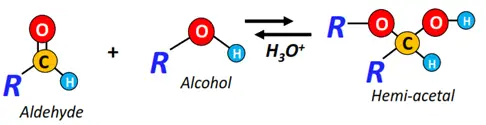
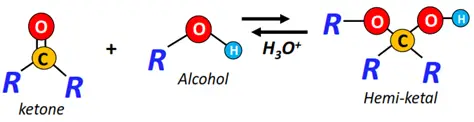
- Nucleophilic addition of alcohols
The reversible addition of alcohols onto aldehydes and ketones gives addition products, i.e., hemiacetals and hemiketals, respectively.
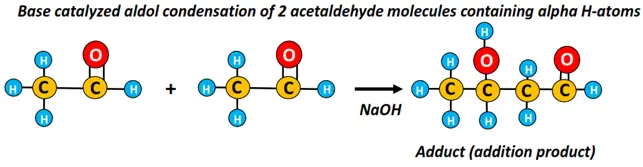
- Aldol condensation
A dimerization reaction occurs when 2 acetaldehyde molecules are treated with 10% NaOH at or below room temperature. It results in the formation of an adduct which is both an aldehyde and an alcohol, simultaneously. Thus, this addition product is known as an aldol. The chemical reaction is called aldol condensation, in which a complex molecule is formed by joining two simple molecules.
Aldol condensation can occur between 2 aldehyde molecules, an aldehyde and a ketone with alpha hydrogen. It can be both acid-catalyzed or base-catalyzed.
- Oxidation reaction
The oxidation of an aldehyde in the presence of a strong oxidizing agent (such as KMnO4) yields a carboxylic acid.
Example: The oxidation of acetaldehyde produces acetic acid (ethanoic acid). Aldehydes can even be oxidized by atmospheric oxygen. This oxidation is known as autoxidation.
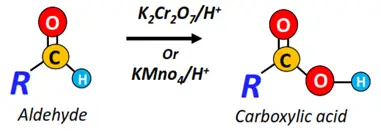
In contrast, ketones do not generally undergo an oxidation reaction as the carbonyl carbon does not carry any H-atom. Under severely oxidative conditions, such as heating at high temperatures in the presence of a strong oxidizing agent, ketones convert into enols which further undergo oxidative cleavage.
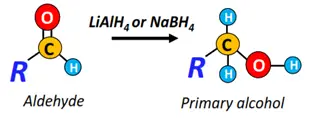
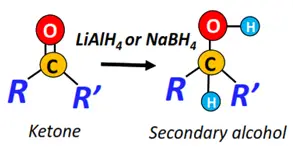
- Reduction reactions
When treated with a reducing agent such as LiAlH4 or NaBH4, aldehydes reduce to primary alcohols, while ketones get reduced to secondary alcohols.
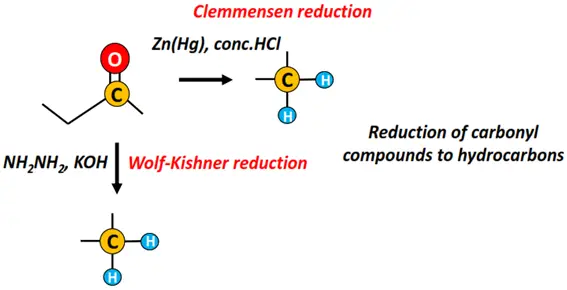
Clemmensen reduction refers to the reduction of aldehydes and ketones into hydrocarbons under reflux conditions in the presence of zinc amalgam (Zn-Hg) and 25% HCl solution.
If aldehydes and ketones are reduced to hydrocarbons by hydrazine (NH2-NH2) and a base (such as KOH), it is then known as Wolf-Kishner reduction.
In both Clemmensen reduction and Wolf-Kishner reduction, the carbonyl (C=O) group is reduced to methylene (-CH2-).
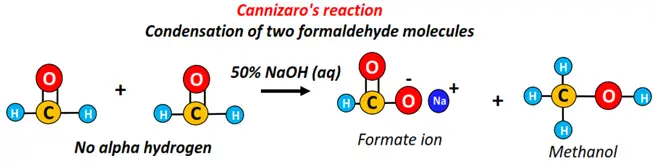
- Cannizzaro’s reaction
In the disproportionation reaction (self-oxidation and reduction), one-half of an aldehyde is oxidized to carboxylic acid. At the same time, the other half is reduced to a primary alcohol, which is known as Cannizzaro’s reaction.
Cannizzaro’s reaction occurs between two aldehydes without alpha-hydrogen and thus cannot undergo aldol condensation. Examples include formaldehyde and benzaldehyde.
The addition of 2 formaldehyde molecules in the presence of 50% NaOH yields sodium formate (HCOO–Na+) and methanol (CH3OH).
For additional help in learning the mechanisms of the above reactions of aldehydes and ketones, you may like this article.
Different types of aldehydes and/or ketones present in a complex sample mixture can be separated via HPLC and GC while characterized through mass spectroscopic (MS) analysis.
Uses of aldehydes and ketones in organic chemistry
As discussed at the beginning of the article, aldehydes and ketones are present naturally in animals, plants and microorganisms.
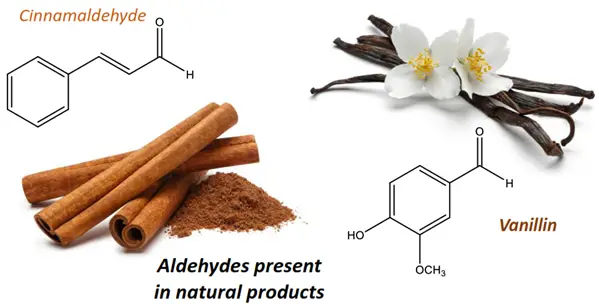
- Aldehydes lend their characteristic flavor and fragrance to substances such as cinnamon (containing cinnamaldehyde) and vanilla bean (containing vanillin). Similarly, ketones such as cortisone functions as an essential hormone in the human body.
- 40% aqueous solution of formaldehyde (formalin) is used to preserve biological specimens.
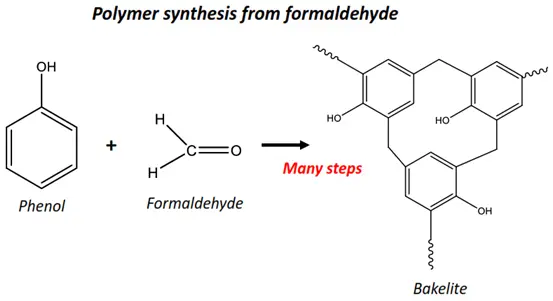
- Formaldehyde or methanal is also used in the polymer industry. It is used for the synthesis of Bakelite, a condensation polymer (resin) composed of formaldehyde and phenol.
- Benzaldehyde (an aromatic aldehyde) is incorporated as an essential component in the cosmetic and perfume industry.
- Ketones such as acetone are used for medicinal purposes, as a paint thinner, nail polish remover, organic solvent, etc.
Find out more interesting uses and applications of organic compounds in a subsequent article.
To revise all the concepts learnt in this article, also check out this detailed video tutorial.
References
1. H. Brown, W. & Poon, T. 2016. Introduction to organic chemistry.
2. M. Younas 2015. A textbook of organic chemistry.
3. Morrison, R. T., Boyd, R. N. & Bhattacharjee, S. K. 2013. Organic chemistry.
4. Sparkman, O. D., Penton, Z. E. & Kitson, F G. 2011. Chapter 9 – Aldehydes. In: Sparkman, O D., Penton, Z. E. & Kitson, F. G. (eds.) Gas chromatography and mass spectrometry (second edition). Amsterdam: Academic Press.
