Alcohols and ethers are extremely valuable organic compounds frequently used in the food, cosmetics, pharmaceutical, flavor and fragrance industries.
In this article, we have explained the chemistry behind alcohols and ethers.
So, for the most valuable and comprehensive information on alcohol and ethers on the web, continue reading the article!
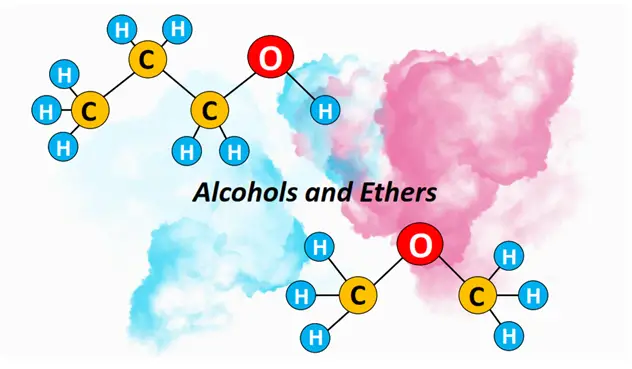
What is alcohol? – Definition & Structure
Alcohols are defined as organic compounds containing a hydroxyl (O-H) functional group. One or more OH groups are covalently bonded to a saturated, aliphatic hydrocarbon chain in an alcohol molecule.
Alcohols are represented by a general formula R-OH or CnH2n+1OH, where R denotes the alkyl chain while n stands for the number of carbon atoms present in the chain.
Methanol (CH3OH), or methyl alcohol, is the first member of the alcohol family possessing 1 carbon atom, followed by ethanol, propanol, butanol, and so on.
Alcohols containing three or more carbon atoms in the alkyl chain, such as propanol, can form different structural isomers based on the position of the OH group.
What is ether? – Definition & Structure
Ethers are organic compounds containing a C-O-C functional group. The C-O-C group is known as the ether linkage. It may be present in an alkyl chain (R) or attached to a phenyl ring (Ar).
Ethers are represented by a general formula CnH2n+2O. Ethers are also known as dialkyl (R-O-R’), alkyl aryl (Ar-O-R), or diaryl (Ar-O-Ar’) derivatives of water.
If the two groups attached on the sides are identical, the ether formed is symmetrical; otherwise, it is referred to as an asymmetrical ether structure.
Examples include dimethyl ether (symmetrical) and ethyl methyl ether (unsymmetrical).
Alcohols and Ethers- nomenclature
Alcohols are generally named by the name of the alkyl group, followed by the word alcohol.
Example: The name methyl alcohol is derived from methane (the alkyl group containing 1 C-atom), followed by adding the word alcohol.
However, the IUPAC name for methyl alcohol is methanol.
For the IUPAC name, the alphabet ‘e’’ is removed from the alkane, and the suffix ‘ol’ is added. In this way, methyl alcohol becomes methanol, ethyl alcohol is ethanol, while propyl alcohol is propanol.
For alcohols having more than one possible structure, i.e., isomers, it is generally named as:
alkan-carbon number carrying OH group-ol.
Example: Propan-1-ol and propan-2-ol are isomers of each other in which the OH group lies at C1 and C2, respectively.
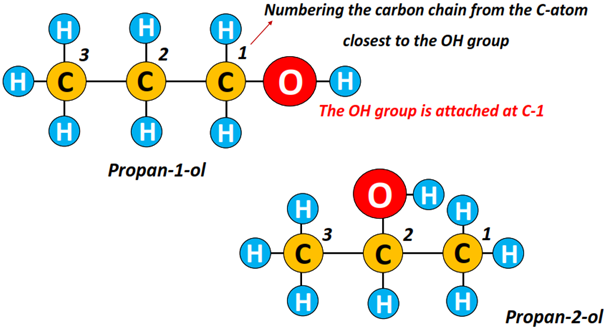
You must remember that the alkyl chain is always numbered starting from the C-atom, which is nearest to the OH functional group.
So, can you name the alcohol structures given below?
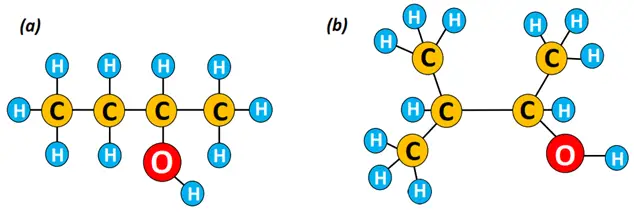
The IUPAC name for structure (a) given above is butan-2-ol, also known as 2-butanol, while that for structure (b) is 3-methyl-butan-2-ol or 3-methyl-2-butanol. In structure (b), the methyl (CH3) group lies at carbon no.3, so the general order of naming is:
Carbon number carrying the methyl or any alkyl group- name of the alkyl group-name of long-chain alcohol.
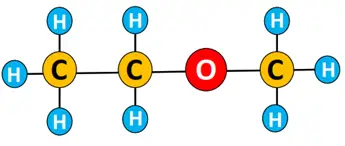
An ether is named following the pattern:
Name of long-chain alkyl on one side of oxygen- name of shorter alkyl chain on the other side of oxygen- ether.
Example: The IUPAC name for the ether given below is ethyl methyl ether.
Alcohols and Ethers- properties
Alcohols:
- Alcohols (R-OH) exists as colorless liquids at r.t.p.
- These are polar solvents possessing an extremely polar hydroxyl (OH) functional group. Oxygen being more electronegative than hydrogen, strongly attracts the O-H bonded electrons towards itself. Oppositely charged poles develop in the molecule, which leads to an overall molecular polarity.
- Alcohols are readily miscible with other polar solvents, such as water, by developing strong hydrogen bonding.
- Increasing the length of the alkyl chain increases the boiling point of the alcohols, while branching reduces the boiling point.
- Alcohols are amphoteric substances, i.e., they can act both as an acid as well as a weak base.
- The acidity of an alcohol molecule is due to the presence of an H-atom bonded to the more electronegative O-atom.
- The basicity of alcohol is accredited to the presence of lone pairs of electrons available for donation on the O-H bonded oxygen atom. Lewis bases are electron pair donors.
Ethers:
- Ethers (R-O-R) exist as sweet-smelling, colorless liquids at r.t.p.
- Ethers are slightly polar molecules, owing to a polar C-O-C linkage.
- However, an ether is less polar than an alcohol of a corresponding chain length due to a smaller electronegativity difference between the bonded atoms in a C-O-C group as opposed to an O-H functional group.
- The boiling point of ethers increases with the increasing number of C-atoms in the alkyl chain.
- Ethers exhibit a slightly basic nature, having lone pairs of electrons on the C-O-C bonded oxygen atom.
Classification of alcohols- primary, secondary and tertiary
Alcohols are primarily classified into primary, secondary and tertiary alcohols.
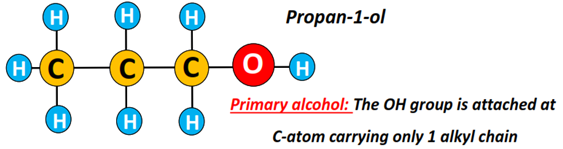
Primary alcohol (1°): The carbon atom carrying the hydroxyl (OH) functional group is attached to 1 other C-atom only.
Examples include methanol, ethanol, propan-1-ol, butan-1-ol, etc.
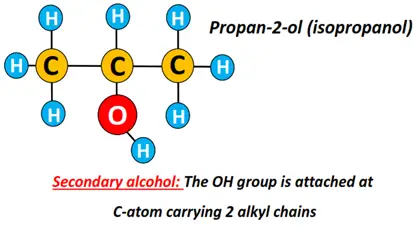
Secondary alcohol (2°): The carbon atom carrying the OH functional group is directly bonded to 2 other C-atoms only.
Examples include propan-2-ol, butan-2-ol, pentan-2-ol.
Propan-2-ol is also known as isopropyl alcohol. The prefix ‘iso’ signifies that two identical CH3 groups are covalently bonded to the C-atom-carrying OH group.
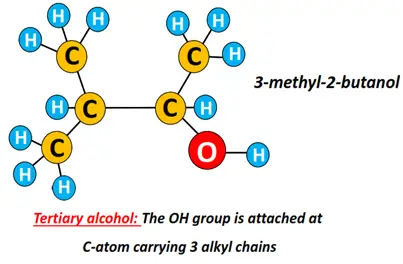
Tertiary alcohol (3°): The carbon atom carrying the OH group is bonded to 3 other C-atoms.
For instance, 3-methyl-2-butanol is a tertiary alcohol.
Tertiary alcohols always form branched structures.
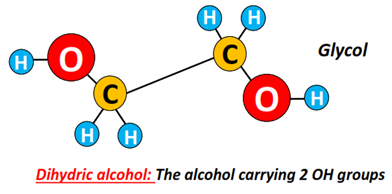
Alcohols can also be classified based on the number of OH groups present in their structures.
Example: An alcohol containing 2 OH groups is known as dihydric alcohol or diol, such as glycol (CH2OH-CH2OH).
Alcohols containing more than 2 OH groups are known as polyols or polyhydric alcohol molecules.
Benzyl alcohol (also known as α-cresol) is an aromatic alcohol as it possesses an OH group bonded to the C-atom, which is directly linked to the phenyl (C6H5) ring.
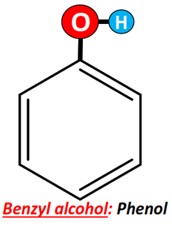
It is important for you to know that if the OH group is directly attached to the benzene ring, in that case, it is not an alcohol molecule. Rather, it is referred to as phenol.
Alcohol: preparation and reactions
Preparation:
On a laboratory scale, alcohols can be prepared from a variety of chemical reactions, including the following:
- Addition reaction of unsaturated hydrocarbons (alkenes)
The addition of a water (H2O) molecule in an alkene forms an alcohol of corresponding chain length. This process is also called hydration.
Example: The chemical reaction of water with ethene (CH2=CH2) breaks down the C=C double bond and ethanol (CH3CH2OH) is formed by adding an H-atom on one C-atom and an OH group on the adjacent C-atom.
- Hydrolysis of alkyl halides
An alkyl halide or halogenoalkane (R-X) is converted into an alcohol molecule under special temperature and pH conditions.
- Reduction of carbonyl compounds (aldehydes and ketones)
Catalytic reduction of an aldehyde produces a primary alcohol, while that of a ketone yields a secondary alcohol.
On an industrial scale, alcohols such as ethanol are prepared by the fermentation of carbohydrates such as glucose.
Reactions:
Reaction of alcohols with metals
An alcohol molecule reacts with an active metal, such as an alkali metal from Group 1 A, to form metal alkoxide and hydrogen gas.
Example: Ethanol reacts with sodium metal to form sodium ethoxide, and H2 is liberated.

Reaction of alcohols with carboxylic acids
The condensation reaction of an alcohol with a carboxylic acid is known as esterification. It produces an ester by releasing a water molecule.
Example: The esterification reaction of ethanol with ethanoic acid (acetic acid) produces ethyl ethanoate (ester) and water.

Oxidation reaction of alcohols
The oxidation of a primary alcohol produces an aldehyde which is further oxidized to a carboxylic acid in the presence of an oxidizing agent (KMnO4 or K2Cr2O7) under acidic or alkaline conditions. The end product is a carboxylic acid molecule under acidic conditions, while a carboxylate ion is produced under alkaline pH.
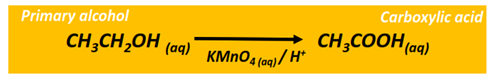
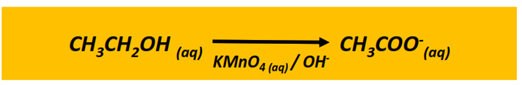
The oxidation of a secondary alcohol produces a ketone.

Dehydration of alcohols
The dehydration of an alcohol produces an unsaturated hydrocarbon (alkene or alkyne). This is known as an elimination reaction.
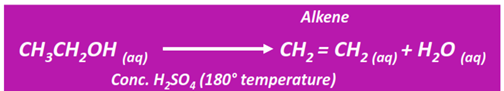
In terms of reactivity, a primary alcohol is more reactive than a secondary alcohol, followed by a tertiary alcohol.

You can distinguish between primary, secondary and tertiary alcohols via Lucas test.
Preparation of ethers from alcohols
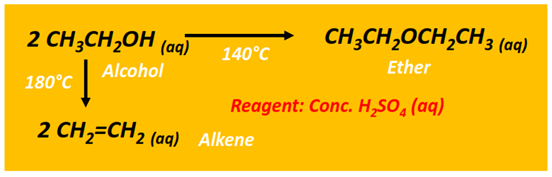
The intermolecular dehydration of an alcohol can also produce an ether. This reaction is carried out in the presence of concentrated sulfuric acid (H2SO4) at 140°C.
Both ethylene and diethyl ether are formed from ethanol dehydration under similar conditions. However, at a lower temperature, symmetrical ether (diethyl ether) is the major product. However, at a relatively higher temperature (180°C), the alkene is recovered as the major product.
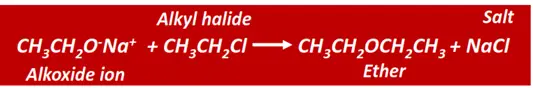
Ethers can also be prepared by Williamson synthesis.
Different functional groups present in an organic compound, such as O-H and C-O-C, can be used for compound identification via infrared (IR) spectroscopy.
Learn what are alcohols and ethers used for in our special article: Uses and applications of organic chemistry.
Also, check out our primary article: Introduction to organic chemistry.
References
1. Ashenhurst, J. 2023. Alcohols to ethers via acid catalysis [online]. [accessed].
2. M. Younas 2015. A textbook of organic chemistry.
3. Morrison, R. T., Boyd, R. N. & Bhattacharjee, S. K. 2013. Organic chemistry.
4. Obodovskiy, I. 2019. Chapter 33 – Basics of Biochemistry. In: Obodovskiy, I. (ed.) Radiation. Elsevier.
