Aromatic organic compounds are more than just sweet-smelling chemical substances, as the word aromatic implies. Rather, they have a fascinating chemistry and a wide range of applications, from perfumes to pharmaceuticals.
Aromatic compounds are also naturally found in fruits and vegetables. The vibrant colors of natural food products are often accredited to the presence of highly conjugated aromatic organic compounds that absorb visible radiations.
In this article, you will learn everything you need to know about aromatic compounds. So let’s dive deeper. Happy learning!
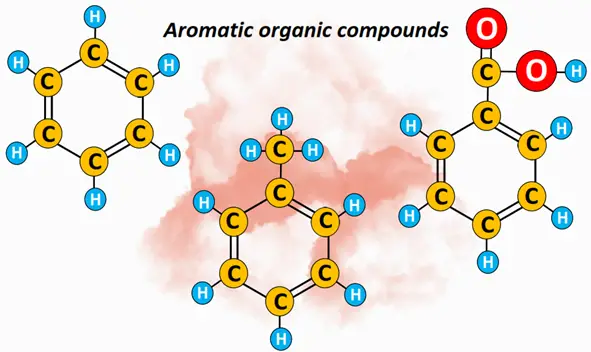
What are aromatic organic compounds? – Definition
Aromatic organic compounds are unsaturated, ringed structures containing a delocalized pi-electron cloud. Benzene (C6H6) is the parent member of the aromatic family. Other aromatic compounds are defined as organic compounds that resemble benzene in their chemical behavior. An aromatic compound must obey Huckle’s rule.
An aromatic organic compound must:
- Be planar
- Contain sp2 hybridized atoms in a continuous ring arrangement.
- Possess complete delocalization of the pi-bonded electrons.
- Follow Huckle’s (4n + 2) π electrons rule where n is an integer (0,1,2,3…).
The chemistry of aromatic compounds- Huckle’s rule of aromaticity
Aromatic compounds are cyclic systems containing delocalized π electrons. A π bond is formed by the parallel overlap of two p-orbitals. The shared electron cloud lies above and below the overlapping region. After bond formation, these atomic orbitals combine to form molecular orbitals.
For the linear combination of atomic orbitals (LCAO) to form molecular orbitals, the atomic orbitals must lie in the same plane, allowing adequate overlap. Hence planarity is an essential requirement for the formation of aromatic compounds.
The bonding electrons present in the molecular orbitals do not stay confined but move from one place to another on the molecule. This movement of π bonded electrons throughout the molecule is known as delocalization.
Aromatic molecules comprise alternate single and double covalent bonds in their rings. The pi-bonded electrons circulate between these bonds, lending extra stability to the molecule.
The presence of alternate single and double bonds in an aromatic organic compound is called conjugation.
The delocalization of electrons over the entire ring results in more than one canonical form (Lewis structure) representing a particular molecule. The actual structure of the aromatic compound is a weighted average of all the possible forms. This concept is thus referred to as resonance. Therefore, aromatic compounds are resonance stabilized.
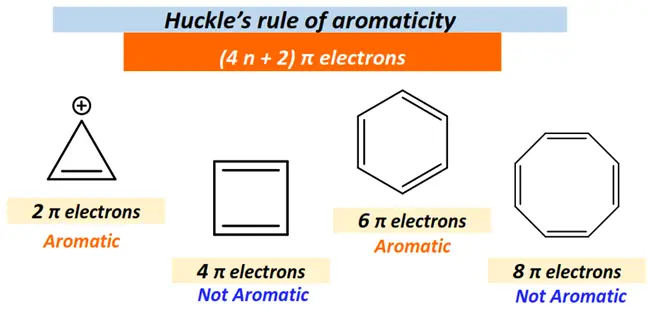
Another prominent feature of aromatic compounds is that they obey Huckle’s rule.
As per Huckle’s (4n + 2) π electrons rule, aromatic compounds contain one or more planar, stable, continuous rings of p-orbitals and π electrons in their cyclic conjugated system equal to 4n + 2, yielding completely filled π molecular orbitals.
For example, Benzene is an aromatic compound consisting of 6π bonded electrons. It is known as [6]-annulene.
(4n + 2) π = 6 π when n =1. Thus, 6 is Huckle’s number. All the C-atoms in the benzene ring are sp2 hybridized.
Contrarily, cyclobutadiene is a cyclic structure, but it is not an aromatic organic compound as it consists of 4π bonded electrons, and 4 is not a Huckle’s number.
No integer value can yield (4n + 2) π = 4π . You can solve it by yourself, as shown below:
4n π + 2 π = 4 π
4n π = 4 π- 2 π
n = 2 π/ 4 π = ½ (not an integer but a fraction)
Cyclohexene readily undergoes electrophilic addition reaction with bromine (Br2), while benzene does not. This exceptional stability of benzene and other such aromatic compounds is referred to as aromatic stabilization. Aromatic stabilization reduces the overall reactivity of the double bond. Hence, aromatic compounds normally undergo substitution reactions instead of addition reactions.
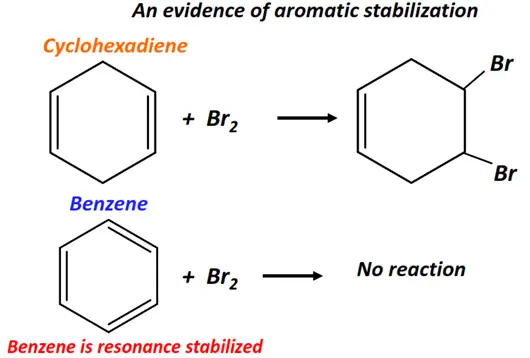
Learn more about the chemistry of benzene in our subsequent article.
Classification of aromatic organic compounds
Aromatic organic compounds are mainly classified into:
- Monoaromatics
- Polyaromatics
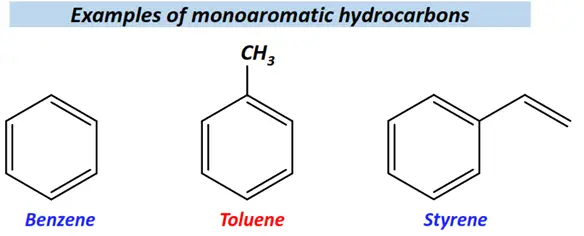
Monoaromatics are aromatic compounds containing a single ring.
For instance, benzene, toluene, styrene, etc., are monoaromatics.
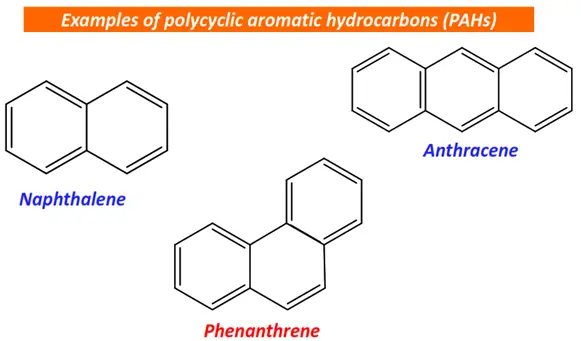
In contrast, polyaromatics contain multiple aromatic rings fused together.
Polycyclic aromatic hydrocarbons (PAHs) are derived from polyaromatics by the incomplete combustion of organic matter. Their examples include naphthalene, phenanthrene, anthracene, etc.
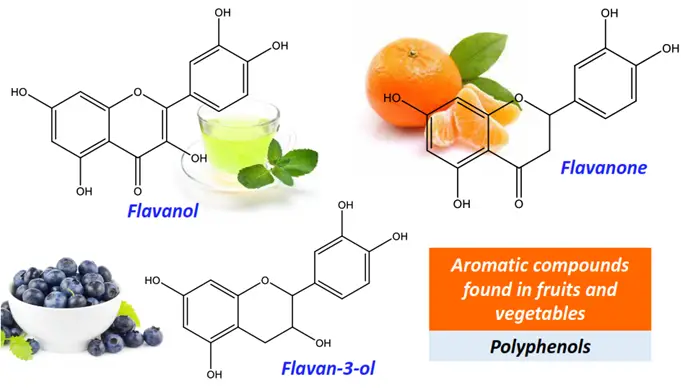
Polyphenols are a subclass of polyaromatics. Polyphenolic aromatic compounds such as flavonoids, flavones, flavanones and anthocyanidins are naturally found in fruits and vegetables. Their antioxidant and anticarcinogenic properties protect against chronic diseases and slow down human aging.
What is a heterocyclic aromatic compound?
Heterocyclic aromatic compounds are organic compounds in which one or more C-atoms in the cyclic backbone are replaced by a hetero atom.
Oxygen (O), nitrogen (N) and sulfur (S) are typical examples of heteroatoms containing lone pairs of electrons.
For example, pyridine is a heterocyclic aromatic compound.
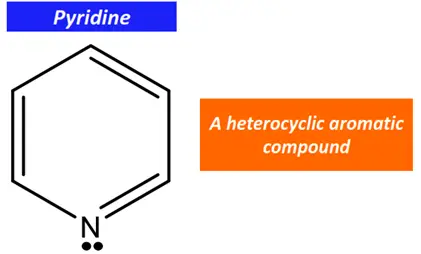
In pyridine, one C-atom in the aromatic ring is replaced by an N-atom. However, the N-atom is sp2 hybridized. Both sp2 hybrid orbitals form covalent chemical bonds with the adjacent C-atoms (2 sigma bonds and a pi bond). The lone pair of electrons stay localized on the N-atom in its unhybridized p-orbital.
Due to the planar arrangement of three sp2 hybrid orbitals of nitrogen, a continuous delocalized pi-electron cloud exists in the pyridine molecule. Furthermore, pyridine follows Huckle’s rule as it consists of a total of 6π electrons. Thus, pyridine is an aromatic compound.
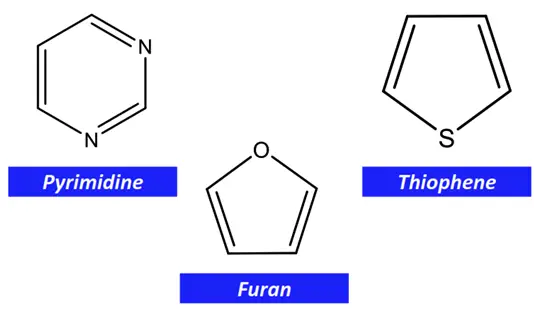
Other examples of heterocyclic aromatic compounds are pyrimidine, furan, thiophene, etc.
What is the difference between aliphatic and aromatic organic compounds?
The main differences between aromatic organic compounds and aliphatic hydrocarbons are outlined below.
| Aliphatic organic compounds | Aromatic organic compounds |
| Straight or branched-chain molecules with open carbon chains | Ringed arrangement of carbon atoms. |
| Geometric or connective arrangement of individual C and H-atoms | Cyclic planar structure with a ring of resonance bonds. |
| Lower stability | Exceptional stability due to delocalization of electrons in a conjugated pi-bonded system. |
| Less dense | Generally more dense due to a closely packed arrangement of C-atoms |
| More reactive | Reduced reactivity due to aromaticity. |
Important aromatic compounds in chemistry
- Aromatic organic compounds such as benzene and its derivatives (phenol, xylene, toluene) are frequently used in the polymer industry for manufacturing plastics and synthetic fibers.
- Important pharmaceutical drugs, such as aspirin, ibuprofen, penicillin, etc., are aromatic compounds.
- Due to their pleasant taste and odor, aromatic compounds such as vanillin, eugenol, and limonene are used as flavor enhancers and to design new fragrances.
- Aromatic compounds are also important in biological functions. For instance, the amino acid phenylalanine, which contains an aromatic ring, acts as a precursor for imminent biomolecules, including dopamine, adrenaline, and thyroid hormones.
- Thymine, a major component of DNA, consists of a heterocyclic aromatic pyrimidine ring.
For additional help on aromatic organic compounds, you make consult this article on aromaticity.
Also, check out these example questions and revise everything you have learned about aromatic organic compounds in this article.
References
1. Morrison, R. T., Boyd, R. N. & Bhattacharjee, S. K. 2013. Organic chemistry.
2. Ouellette, R. J. & Rawn, J. D. 2015. 5 – Aromatic compounds. In: Ouellette, R. J. & Rawn, J. D. (eds.) Principles of organic chemistry. Boston: Elsevier.
