Organic reactions have revolutionized modern science. From the synthesis of life-saving drugs to the creation of innovative materials, organic chemistry and its reactions play a pivotal role. It is due to the unique properties of carbon that it can form multiple bonds, chains, and rings and thus versatile organic compounds.
In this article, we will explore the 6 main types of reactions in organic chemistry, their mechanisms, specificity and applications, so come along and continue reading!
What is an organic chemistry reaction?
Organic reactions involve breaking and making chemical bonds between carbon and other atoms, such as hydrogen, nitrogen, oxygen, sulfur, halogens, etc. The organic reactions are usually carried out using inorganic compounds as reagents, while the organic compounds undergoing the chemical transformation are known as substrates. The reagents include oxidizing and reducing agents and Lewis acids and bases used as catalysts.
‘’Reagent + Substrate = Product’
What are the different types of reactions in organic chemistry?
All the organic reactions can be classified into the following 6 main types and sub-types.
Organic reactions’ mechanisms & their Examples
i) Substitution reactions
In a substitution reaction, a functional group or an atom of the substrate is removed and replaced by another atom or group of the reagent.
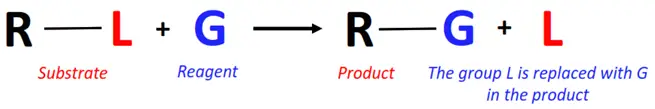
Substitution reactions can be further classified into:
Free radical substitution
Free radicals are highly reactive, unstable chemical species possessing unpaired electrons. Free radical substitution involves the replacement of an atom or a group of atoms from the organic molecule with a free radical.
Free radical substitution mainly supports the halogenation reaction of saturated hydrocarbons; alkanes. It comprises the following main steps:
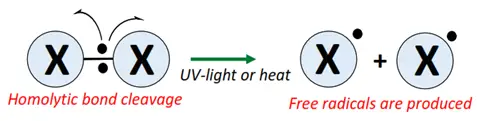
- Initiation: The halogen molecule undergoes homolytic bond cleavage in UV light or heat (250-400 °C), generating free radicals (X•).
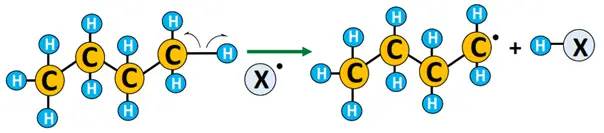
- Chain propagation: The free radical reacts with the substrate by abstracting a hydrogen atom from it and transforming it into a free radical (R•). The radical, in turn, knocks out an electron from another X2 molecule, leading to a chain reaction.
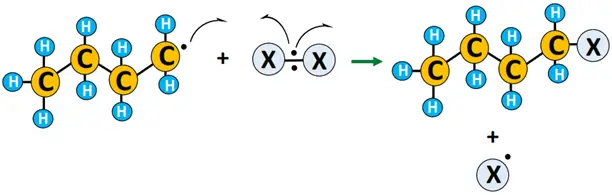
- Termination: X• combines with R• to yield the required product, i.e., alkyl halide (R-X). 2 R• combines to form R2, terminating the reaction.
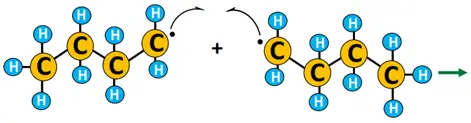
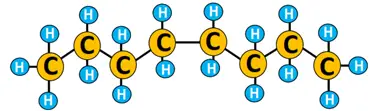
Example of free radical substitution
The chlorination of propane (CH3CH2CH3) to form 1-chloropropane.
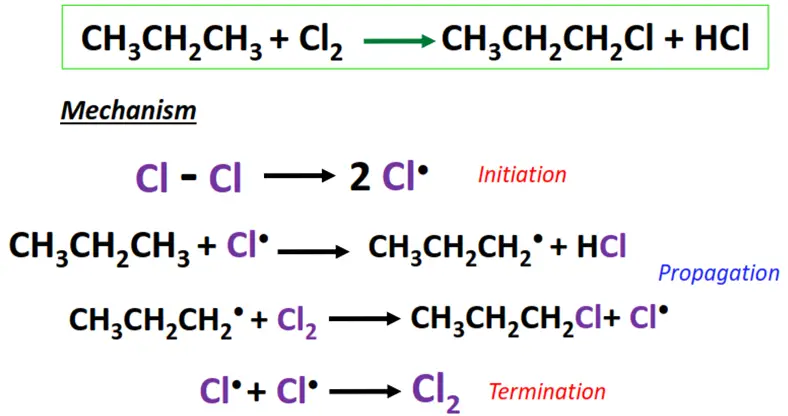
Free radical substitution reactions are primarily useful in polymerizations and environmental pollutants’ degradation.
Nucleophilic substitution
Three main components of nucleophilic substitution reactions are:
- Substrate
- Nucleophile
- Solvent
The substrate consists of two parts, i.e., the alkyl group and leaving group.
Nucleophiles are electron-rich species having a lone pair of electrons or pi-bonded electrons that can be used to form a new chemical bond.
Lewis bases form good nucleophiles. Some examples of good nucleophiles are anions such as OH–, CN– and halides (Cl–, Br–, I–), etc. Water (H2O) can act as a nucleophile in certain chemical reactions by donating the 2 lone pairs of electrons present on its O-atom.
In a nucleophilic substitution reaction:
- The nucleophile (Nu) attacks the substrate on an electron-deficient center, such as a C-atom bonded to a leaving group.
- A transition state is achieved in which the Nu is temporarily attracted to the substrate while the leaving group departs.
- The final product is formed as the nucleophile displaces the leaving group by forming a bond with the alkyl group.
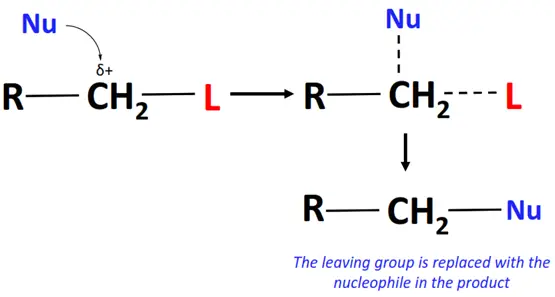
Nucleophilic substitution reactions can be subdivided into nucleophilic aliphatic and nucleophilic aromatic substitutions depending upon the substrate involved.
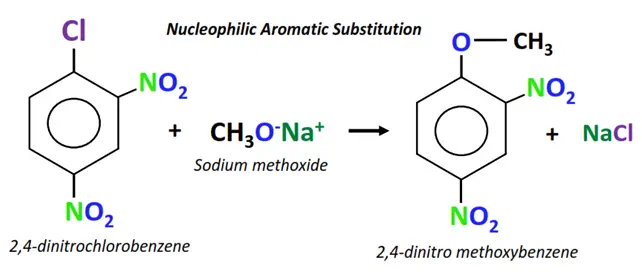
The chemical reaction of an alkyl halide to form alcohol is an example of nucleophilic aliphatic substitution. Meanwhile, nucleophilic aromatic substitutions are rare but possible. For instance, the organic reaction between 2,4-dinitro chlorobenzene and sodium methoxide is an example of nucleophilic aromatic substitution.
Example of nucleophilic aliphatic substitution
The hydrolysis of 1-chloropropane.
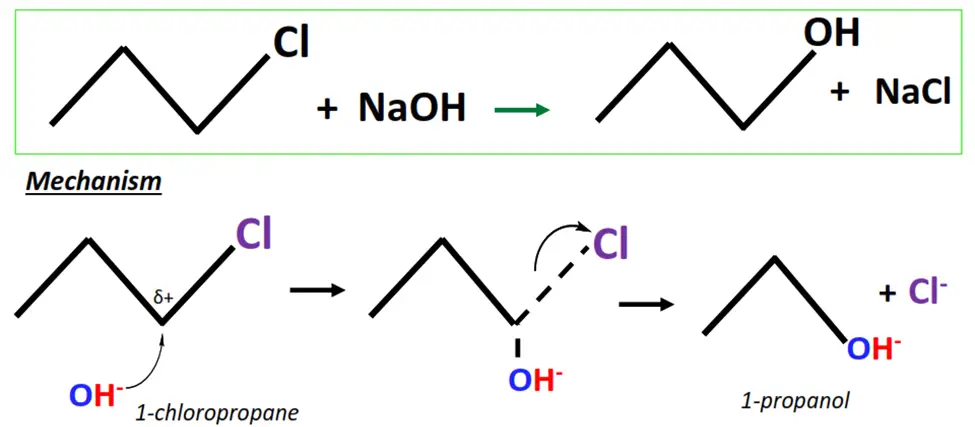
- OH– ions act as a nucleophile and attack the Cδ+ center of 1-chloropropane (substrate).
- A transition state is achieved as the OH– ion temporarily attaches to the substrate while the leaving group (Cl) is weakly held.
- The C-Cl bond breaks while a new C-O bond is formed, resulting in the final product, i.e., 1-propanol.
You may note that the above example follows the SN-2 mechanism of nucleophilic substitution, as the substrate involved is a primary alkyl halide. For tertiary alkyl halides, nucleophilic substitution takes place via the SN-1 mechanism.
Learn more about SN-1 and SN-2 mechanisms in our special article on alkyl halides and aryl halides.
Example of nucleophilic aromatic substitution
The chemical reaction between 2,4-dinitro chlorobenzene and sodium methoxide.
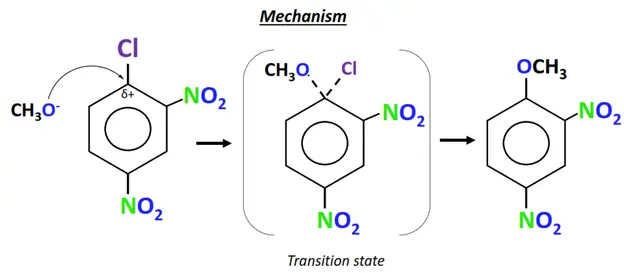
- The methoxide ion (CH3O–) acts as the nucleophile. It attacks the electron-deficient C-atom directly bonded to the Cl-atom (leaving group).
- The C-Cl bond breaks and a new C-O bond is formed on the same carbon.
- Cl– departs the molecule and is replaced by OCH3.
- A new aromatic compound is obtained as the final product, while NaCl is released as an end product.
Electrophilic substitution
The mechanism of electrophilic substitution reactions is the reverse of nucleophilic substitutions. In this case, an electron-deficient species, such as a cation, attacks an electron-rich site on the organic molecule, consequently replacing the leaving group and forming a new molecule. Lewis acids (AlCl3, FeBr3), H+ ions, and carbocation (R+) are some examples of good electrophiles.
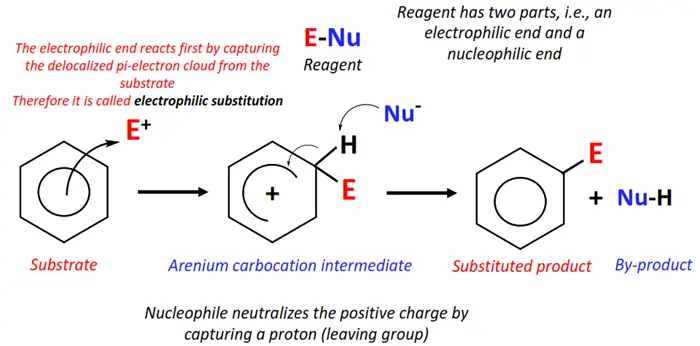
Electrophilic substitutions predominantly support the substitution reactions of aromatic compounds, i.e., benzene and its derivatives.
Halogenation, nitration, sulphonation and hydrolysis of benzene and aryl halides all fall under electrophilic substitution reactions.
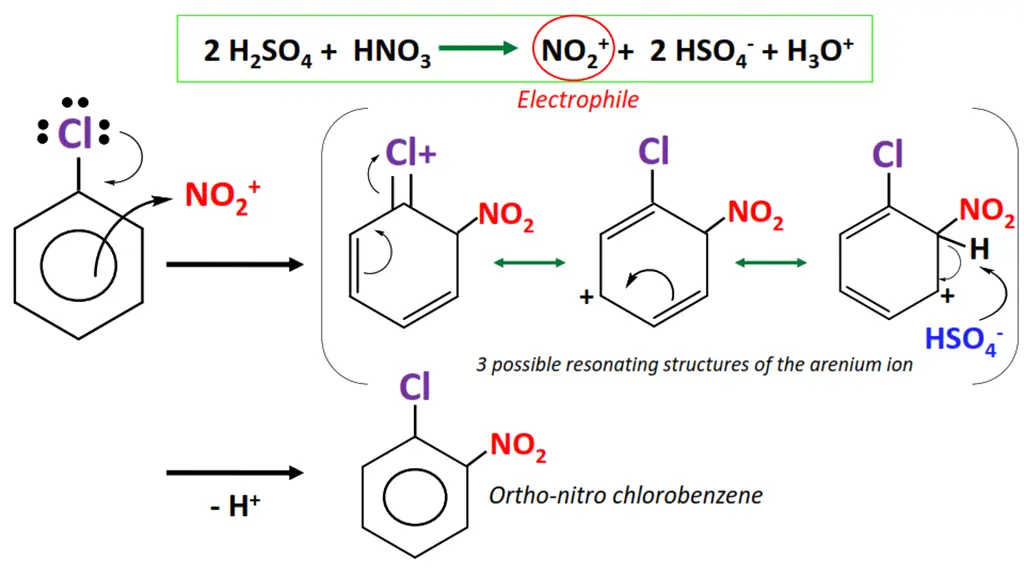
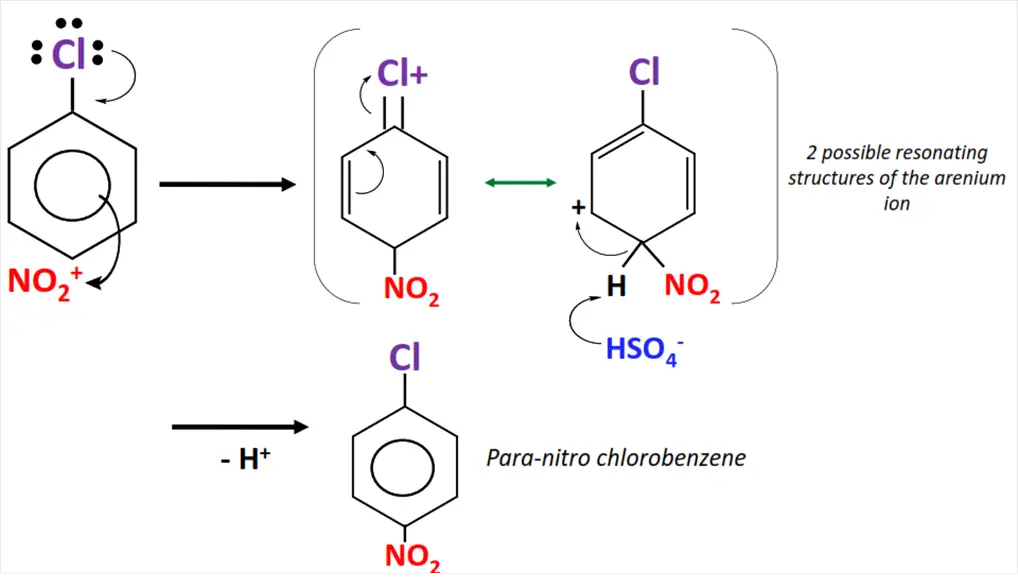
Example of electrophilic substitution
The nitration of chlorobenzene.
- Sulfuric acid (H2SO4) reacts with nitric acid (HNO3) to generate an electrophile i.e., the nitronium (NO2+) ion.
- NO2+ attacks the delocalized pi-electron cloud in chlorobenzene, preferably from ortho and para positions.
- The C=C bond breaks as NO2+ is attached to the aromatic ring, resulting in an unstable intermediate called an arenium ion.
- The arenium ion loses a proton, re-stabilizing the pi-electron cloud within the ring.
- o-nitro chlorobenzene and p-nitro chlorobenzene are obtained as major products in this organic reaction.
ii) Addition reactions
In an addition reaction, the ringed structures or multiple (C=C or C≡C) bonds present in the substrate break while one or two molecules of the reagent are added to it.
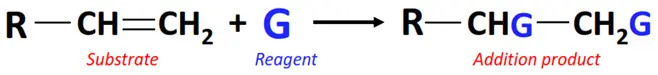
Additions reactions are further classified into the following main types:
Electrophilic addition
In an electrophilic addition reaction, an electron-deficient reagent (electrophile, E+) attacks the electron-rich site of an unsaturated molecule. The multiple bonds break into single, and a saturated organic molecule is obtained by forming new covalent bonds with the electrophile.
Examples of electrophilic addition reaction
1.The hydrogenation of ethene
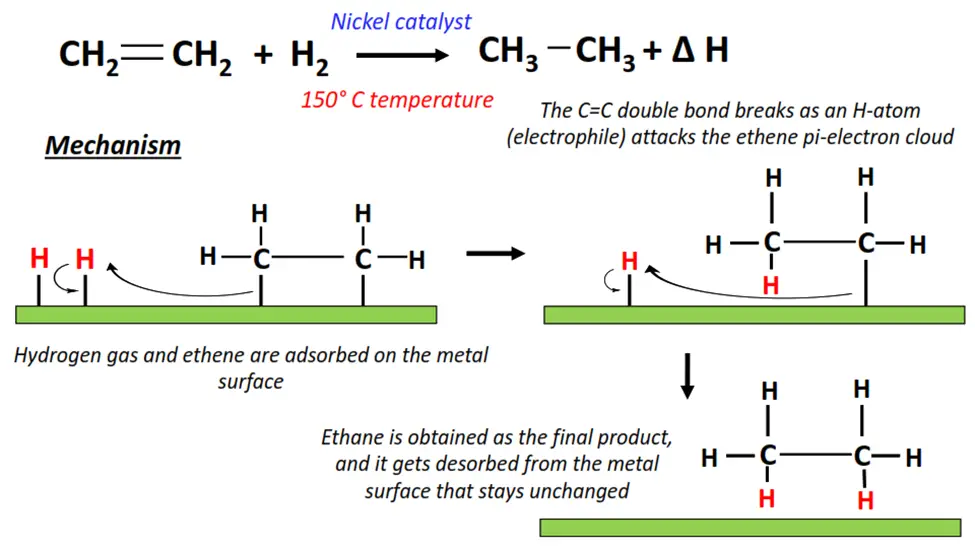
- Hydrogenation is an exothermic reaction that occurs in the presence of H2(g) as a reagent while metals (such as Ni, Rh, and Ir) are used as catalysts.
- H2 attacks the C=C double bond in CH2=CH2.
- The C=C double bond breaks into a C-C single bond, adding an H-atom to each adjacent C-atom.
- Ethane (CH3-CH3) is recovered as the final product. The energy released during the reaction is known as the heat of hydrogenation (∆H).
- The reaction occurs on the surface of the metal catalyst; however, once the reaction reaches completion, the catalyst is recovered unchanged and ready to reuse.
Electrophilic addition reactions involving asymmetric substrates and reagents such as propene and H2O offer regioselectivity as they obey the Markovnikov rule.
2. Propene hydration
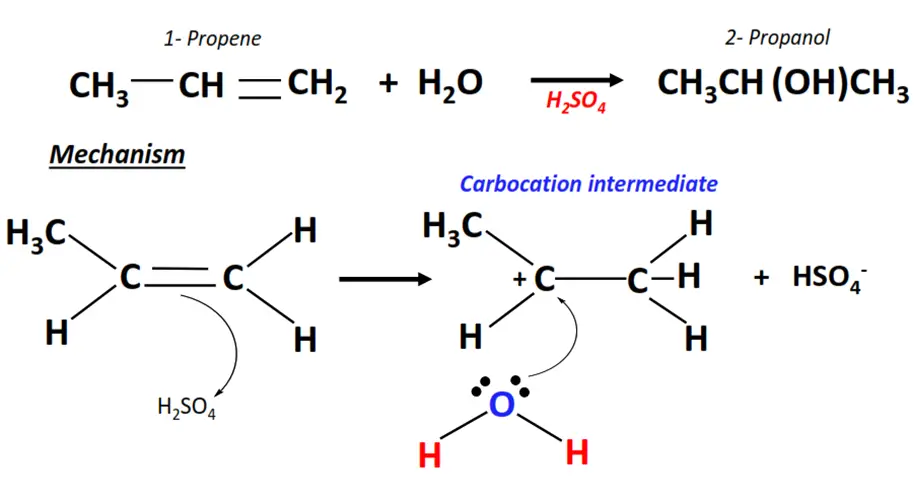
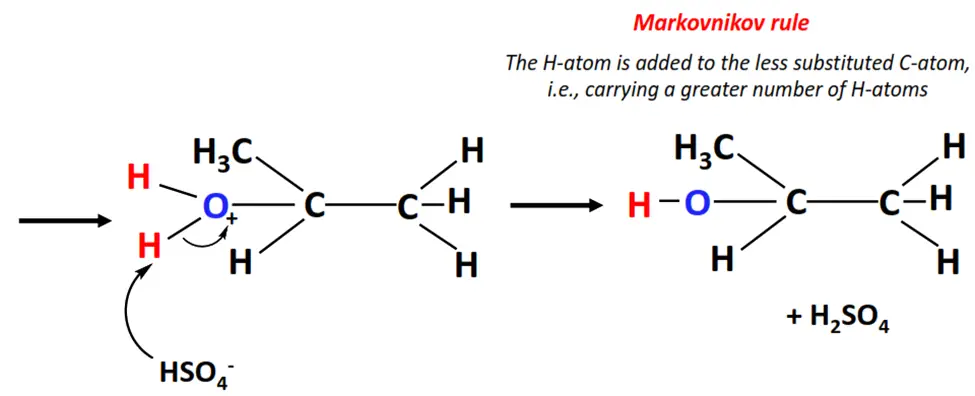
- Hydration refers to the acid-catalyzed electrophilic addition of water to an alkene molecule such as propene (CH3-CH=CH2).
- The H+ ion acts as an electrophile. It attacks the C=C bond and gets attached to it temporarily, generating a carbocation.
- The OH– ion (a nucleophile) then attacks the positively charged center on the carbocation and neutralizes its charge.
- In this way, the C=C bond breaks to C-C single bond and an H-atom and an OH group are added to the adjacent C-atoms, yielding 2-propanol, as the major product.
Nucleophilic addition
Nucleophilic addition reactions usually occur in carbonyl compounds, i.e., aldehydes and ketones possessing an electron-deficient C-atom involved in the C=O bond. The nucleophile attacks the electrophilic C-atom. The C=O bond breaks, forming two new bonds and, thus, the final product.
Example of nucleophilic addition
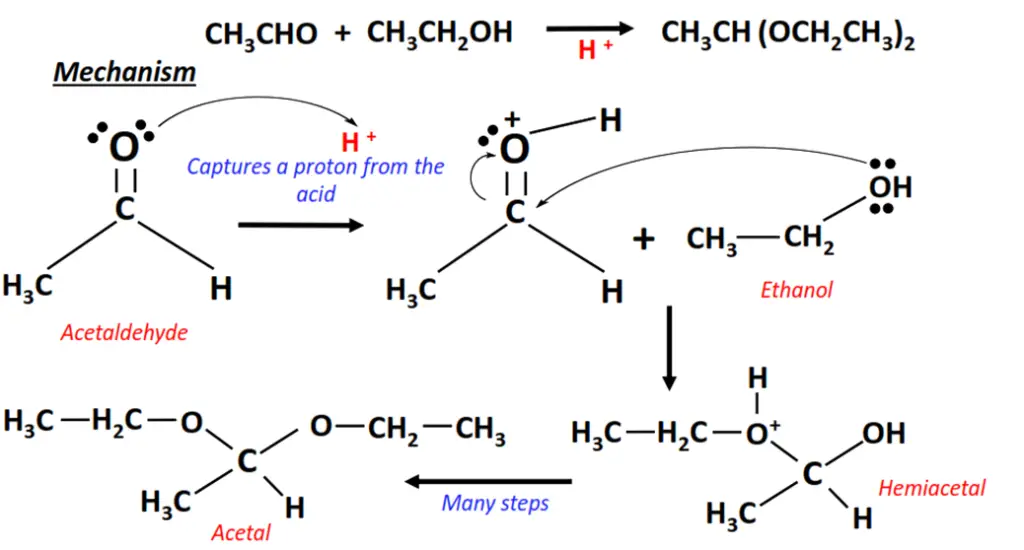
The addition of ethanol to acetaldehyde.
- The carbonyl group gets protonated as the lone pairs of electrons present on C=O capture a proton (H+) from the anhydrous acid.
- The ethanol molecule uses its lone pair to attack the C=O bonded carbon atom and, in turn, get attached to it.
- H2O captures an H+ ion from this intermediate. The intermediate gets deprotonated to form a hemiacetal.
- The hemiacetal is finally converted into acetal after a series of steps in between.
- As a final result, the C=O bond breaks into C-O; two reagent molecules (OC2H5 and C2H5) are added to the substrate, one on each side.
iii) Elimination reactions
The elimination reaction is a type of organic reaction in which a molecule loses atoms or functional groups from adjacent C-atoms, resulting in the formation of multiple (double or triple) bonds. The displaced atoms combine and are released as a by-product.
Elimination reactions follow two different mechanisms, i.e., E1 and E2.
E1 is a slow and less efficient process as compared to E2. The E2 mechanism is preferred for primary and secondary substrates, and it proceeds via a carbocation intermediate, unlike E1, which is a single-step process preferred for tertiary substrates.
Find out more about E1 and E2 type elimination reactions here.
Examples of elimination reaction
1.Dehydrohalogenation of 2-bromobutane
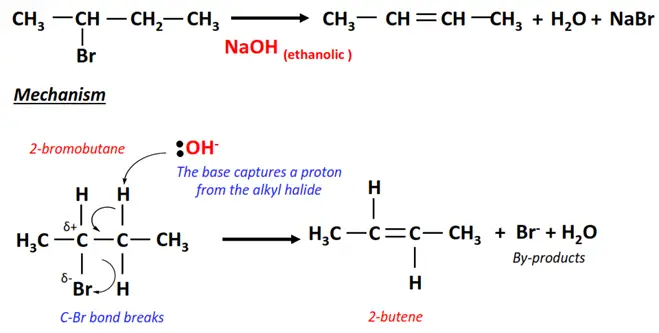
- In the presence of ethanolic NaOH, the Br-atom is removed from the alpha carbon of 2-bromobutane while an H-atom is removed from an adjacent carbon.
- A C=C double bond is formed between the two C-atoms.
- 2-butene is the major product, while 1-butene is the minor product in this elimination reaction, as it follows Saytzeff’s rule.
- NaBr and H2O are obtained as by-products.
Saytzeff’s (or Zaitsev’s) rule states that during an elimination reaction, the most substituted product is the more stable and, thus, the preferred one. So, out of the two possibilities in 2-bromobutane, the H-atom is removed from a C-atom with fewer H-atoms already.
Pro-tip: Treating an alkyl halide with aq NaOH results in a nucleophilic substitution reaction; contrarily, if ethanolic NaOH is used, an elimination reaction occurs.
2. Dehydration of 3-methyl-butan-2-ol
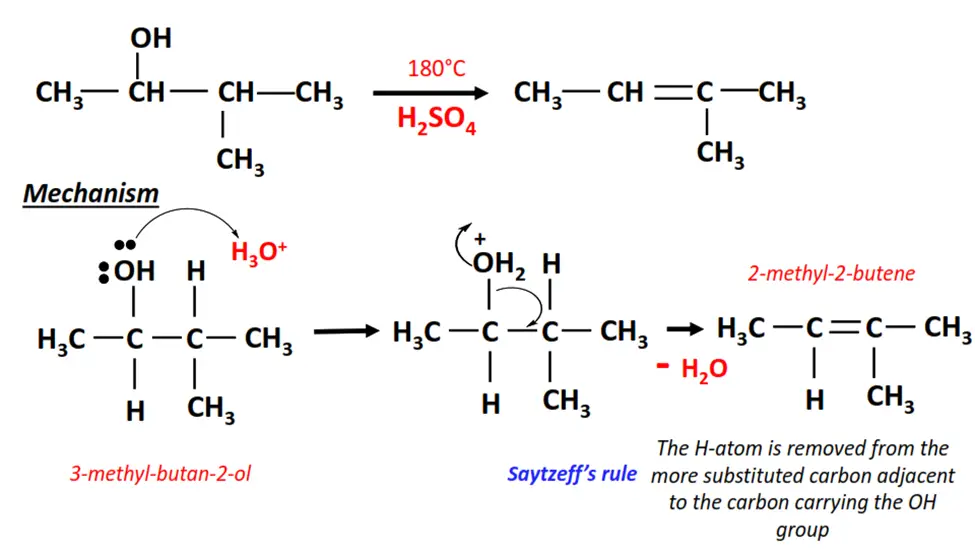
- In the presence of an acid, the OH group is removed from C-2 on 3-methyl-butan-2-ol, and an H-atom is removed from the adjacent carbon.
- A C=C double bond is formed between the two carbon atoms, yielding a mixture of products, 2-methyl-2-butene being the major product.
- H2O is released as a by-product of this elimination reaction.
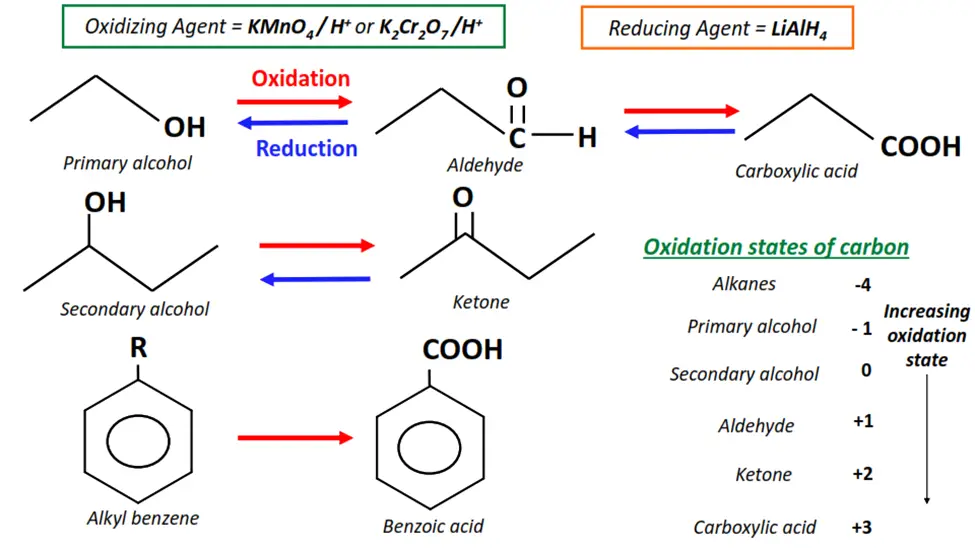
iv) Redox reactions
In the redox reactions, also known as the oxidation-reduction reactions, the reagent changes the oxidation number of the C-atom in the substrate molecule.
Oxidation denotes the lowering of the oxidation number, while reduction refers to an increase in the oxidation number. If the reagent oxidizes the C-atom in the substrate, it gets reduced itself and vice versa.
Examples of redox reactions in organic chemistry
- The oxidation of primary alcohols to aldehydes and carboxylic acids.
- The oxidation of secondary alcohols to ketones.
- The oxidation of aldehydes to carboxylic acids.
- The oxidation of alkyl functional groups on an aromatic ring to carboxylic acids.
- The reduction of aldehydes and ketones to primary and secondary alcohols, respectively.
v) Rearrangements
In an organic rearrangement, the bond connectivity within the molecule changes, resulting in a different organic compound having the same molecular formula. The change in bond connectivity occurs due to the migration of an atom or a functional group from one location to another on the molecule.
The two main types of rearrangements in organic chemistry are:
Examples of organic rearrangements
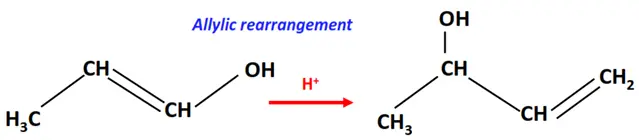
1.Allylic rearrangement
An allyl group refers to a substituent having the structural formula –CH2-CH=CH2–
In the allylic rearrangement, the double bond in the allyl group shifts to the next carbon atoms.
2. Wagner-Meerwein rearrangement
In the Wagner-Meerwein rearrangement, an H-atom, an alkyl (R) group or an aryl (Ar) group migrates from one carbon to a neighboring carbon atom on the molecule.
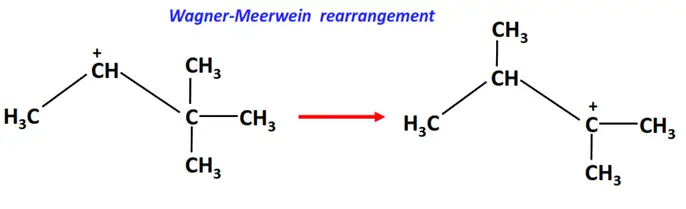
vi) Pericyclic reactions
Pericyclic reactions refer to the electronic reorganization of an organic molecule mediated through a transition state. One or more bonds are broken while new bonds are formed; the bonding changes cyclically.
Examples of pericyclic reactions
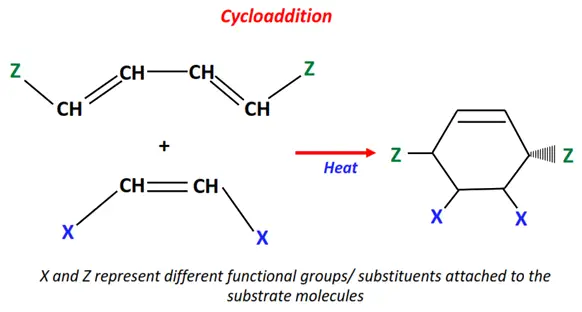
1.Cycloaddition reaction
Two organic molecules undergo cycloaddition in the presence of heat or electromagnetic radiation.
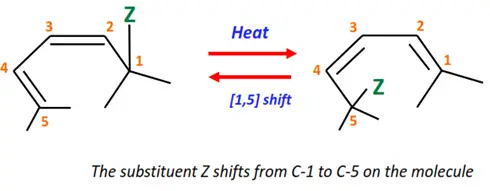
2.Sigmatropic reaction
In this type of pericyclic reaction, a sigma bond between two adjacent atoms breaks, resulting in a new sigma bond between the same two atoms but in a different arrangement.
Shown below is the [1,5]-sigmatropic reaction in which the substituent moves from position 1 to position 5 of the conjugated diene.
All the above organic reactions are extremely useful in research and development and in synthesizing novel drugs, medicines, polymers and therapeutic agents. You may learn more about that in our article on different uses and applications of organic chemistry.
If you want to make a mind map of all the organic reactions taught in this article, there is nothing better than keeping a reaction cheat sheet.
Also, revise your concepts through this video lecture.
References
1.H. Brown, W. & Poon, T. 2016. Introduction to organic chemistry.
2. M.Younas 2015. A textbook of organic chemistry.
3. Morrison, R. T., Boyd, R. N. & Bhattacharjee, S. K. 2013. Organic chemistry.
4. Savin, K. 2014. Writing reaction mechanisms in organic chemistry, 3rd edition.
