Hydrocarbons are the simplest organic compounds. Organic hydrocarbons are binary compounds predominantly present in natural gas, diesel, fuel, and kerosene oil. The length of a hydrocarbon chain strongly influences its physicochemical properties and, thus, its usage.
In this article, we have discussed everything about organic hydrocarbons, so without any further delay, dive into the article and start reading!
What are hydrocarbons?
The name hydrocarbon can be split into words, i.e., hydro and carbon. Here hydro refers to the element hydrogen (H), while carbon denotes the tetravalent element carbon (C), which is an essential component of all organic compounds.

Definition
Hydrocarbons are defined as organic compounds made up of only two elements, i.e., carbon (C) and hydrogen (H). The C-atoms get interlinked via covalent bonding to form the basic skeleton of a hydrocarbon. The H-atoms then get attached to the hydrophobic carbon chain in different configurations to satisfy the valency of each C-atom. This yields an overall non-polar hydrocarbon chain.
Single, double or triple covalent bonds can be present among the interlinked C-atoms. However, the C-H bond is always a single covalent bond in which the C-atom attains a complete octet while the corresponding H-atom completes its duplet electronic configuration.

Classification of hydrocarbons
On the basis of structure, hydrocarbons can be classified into:
- Aliphatic hydrocarbons
- Alicyclic hydrocarbons
On the basis of the type of covalent bonds present, hydrocarbons can be classified into:
- Saturated hydrocarbons
- Unsaturated hydrocarbons

Saturated and Unsaturated hydrocarbons
Saturated hydrocarbons contain all C-C single covalent bonds. There is no vacancy for accommodating other atoms in the long alkyl chain.
Unsaturated hydrocarbons are comprised of C=C double or C≡C triple covalent bonds in between C-C single bonds in the long hydrocarbon chain.
C=C or C≡C bonds contain delocalized electrons, so these are easier to break and accommodate new atoms or functional groups. Consequently, unsaturated hydrocarbons are generally more reactive than saturated hydrocarbons, possessing lower melting and boiling points than the latter.
Aliphatic hydrocarbons are open-chain structures. These are further divided into:
- Alkanes
- Alkenes
- Alkynes
- Cycloalkanes
Alkanes, alkenes and alkynes
Alkanes
Alkanes are saturated hydrocarbons containing all C-C single covalent bonds. These are represented by a homologous series containing a general formula, i.e., CnH2n+2, where n= number of carbon atoms in the hydrocarbon.
Each C-atom in an alkane molecule is sp3 hybridized. Therefore, the shape of the molecule w.r.t each C-atom is tetrahedral. Each C-atom is bonded to two other C-atoms, one on each side and two H-atoms, one above and the other below the central C-atom.
Methane (CH4), the first member of the alkane family, is the simplest organic compound, followed by ethane (C2H6), propane (C3H8), butane (C4H10), pentane (C5H12), etc.
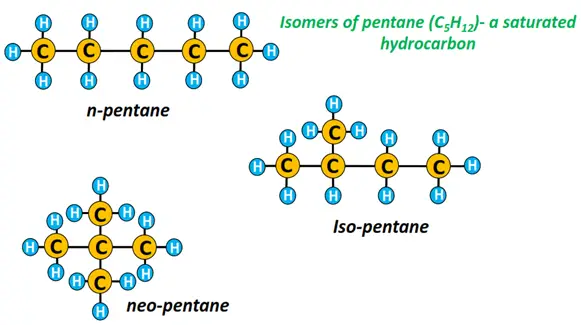
Alkanes can form straight-chain structures such as n-pentane, where the prefix n denotes normal, i.e., a linear hydrocarbon chain.
Conversely, alkanes can also form branched chains, such as isopentane (IUPAC name: 2-methylbutane) and neopentane (IUPAC name: 2,2-dimethylpropane). n-pentane, isopentane and neopentane are three different isomers of pentane, an alkane containing five carbon atoms.
Non-polar alkane molecules are held together by weak van der Waal’s forces of attraction (London dispersion forces). The strength of the attractive force in a hydrocarbon chain increase as the size of the molecule increases. That is why short-chain alkanes (C1 to C4) are colorless gases, mid-length alkanes (C5 to C17) are liquids, while long-chain alkanes molecules (> C18) are solids at r.t.p.
Temporary dipoles get induced in a long hydrocarbon chain as the slightly more electronegative C-atom (E.N = 2.55) attracts C-H bonded electrons to a greater extent than the less electronegative neighboring H-atom (E.N = 2.20). Carbon gains a temporary partial negative (Cδ-) charge while the corresponding H-atom obtain a partial positive (Hδ+) charge.
A large number of C-H bonds held together in a long alkane chain means a greater number of temporary dipoles, thus a stronger attractive force. Hence, the molecule possesses a higher melting and/or boiling point as well as density.
However, the molecule stays non-polar, no matter how long or short is the hydrocarbon chain, as equal C-H dipole moments get cancelled uniformly in opposite directions. Therefore, all alkanes are insoluble in polar solvents such as water, as like-dissolves-like. Rather liquid alkanes can be used as non-polar solvents themselves.
You must note that a straight-chain alkane is always more stable with higher melting and boiling points than a branched alkane molecule with the same number of C-atoms. Branching decreases the exposed surface area; consequently, the strength of Vander Waal’s forces of attraction decreases.
∴ Boiling point (n-pentane = 36.1 °C) > Boiling point (neo-pentane = 9.5°C).
Different-length hydrocarbons are obtained by the cracking (thermal decomposition) of long-chain alkane molecules, particularly of petroleum origin.
Alkenes
Alkenes are unsaturated hydrocarbons represented by a general formula, i.e., CnH2n. One or more C=C double covalent bonds are present between C-C single bonds in an alkene molecule. Each double-bonded C-atom is sp2 hybridized in an alkene molecule. Thus, the molecule possesses a trigonal planar shape w.r.t this C-atom.
Ethene (C2H4) is the first member of the alkene family, followed by propene (C3H6), also known as prop-1-ene, as the double bond is present between C1 and C2, followed by butene (C4H8). Butene consists of 3 positional isomers, i.e., but-1-ene (or 1-butene), but-2-ene (or 2-butene) and isobutene (IUPAC name: 2-methyl-propene).

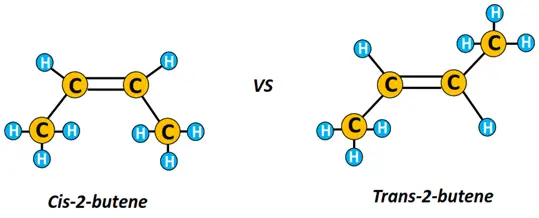
1-butene and 2-butene are straight-chain structures, while isobutene is a branched hydrocarbon. Furthermore, cis-trans geometrical isomerism exists in 2-butene. Cis-2-butene and trans-2-butene are geometrical isomers where trans-2-butene is more stable than cis-2-butene as the former has a lower steric strain.
Alkynes
Alkynes are unsaturated hydrocarbons containing C≡C triple bonds in their molecules. They are represented by a general formula CnH2n-2. Acetylene (C2H2) (IUPAC name: ethyne) is the simplest alkyne molecule.
A C≡C triple covalent bond consists of 1 sigma (σ) and 2 pi (𝛑) bonds, respectively. It is stronger and thus shorter than a C=C double bond which in turn is shorter than a C-C single covalent bond.
Each triple-bonded C-atom is sp hybridized in an alkyne molecule. Thus, the molecule possesses a linear shape w.r.t this C-atom.
Similar to the alkane family, the density, melting point and boiling point of alkenes and alkynes increases as the number of carbon atoms present in a molecule increase.
Alkenes and alkynes can be converted into alkanes via hydrogenation (addition reaction).
Cyclic aliphatic alkanes or Cycloalkanes
Cycloalkanes comprise C-C single bonds arranged as a ring. The general formula of a cycloalkane is CnH2n. These are also sometimes referred to as carbocyclic hydrocarbons. Examples of cycloalkanes include cyclopropane (the simplest cycloalkane), cyclobutane, cyclopentane, cyclohexane, etc.
Cyclohexane (C6H12), cyclooctane (C8H16) and cyclononane (C9H18) are stable cyclic hydrocarbons. However, cyclopropane and cyclobutane are not very stable owing to a higher ring strain due to a smaller number of C-atoms present.
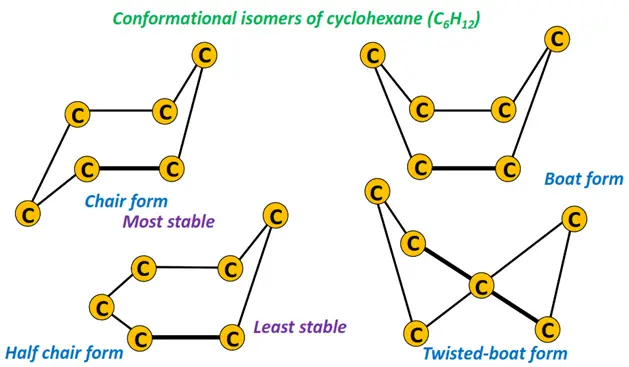
Cyclohexane can adopt different conformations (the boat, the twist-boat, the chair and the half-chair). The chair is the most stable conformation of cyclohexane, as the ring strain is minimized in it.
These different conformations of a cycloalkane molecule are known as conformational isomers or conformers.
Conformers are formed by the continuous rotation of bonds in a cyclic structure without bond breaking.
Alicyclic hydrocarbons are ringed structures in which the interlinked carbon chain is closed. These are further divided into:
- Homocyclic (aromatic, non-aromatic or anti-aromatic)
- Heterocyclic
Homocyclic aromatic hydrocarbons are special kinds of planar, ringed structures possessing a single type of ring.
Aromatic hydrocarbons follow Huckle’s rule (4n + 2 𝛑) electrons. These include benzene and benzene-like chemical compounds that resemble the former in behaviour.
Read more about benzene, the special compound, in our article: The chemistry of benzene.
What is the difference between alkanes, alkenes and alkynes?
| Alkanes | Alkenes | Alkynes | |
| General formula | CnH2n+2 | CnH2n | CnH2n-2 |
| Saturated or Unsaturated | Saturated | Unsaturated | Unsaturated |
| Structure | Contains all C-C bonds in the hydrocarbon skeleton | Contains one or more C=C bonds in between C-C bonds in the hydrocarbon chain | Contains C≡C bonds in between C-C single bonds in the hydrocarbon chain |
| Hybridization | All sp3 hybridized C-atoms | C=C bonded carbon atoms are sp2 hybridized | C≡C bonded carbon atoms are sp hybridized |
| Types of covalent bonding | All C-C bonds are sigma bonds | The C=C bond consists of 1 sigma + 1 pi bond | The C≡C consists of 1 sigma + 2 pi bonds |
| Reactivity | Low | High | Higher |
Different fractions of a hydrocarbon mixture can be separated via fractional distillation, based on the difference in their boiling points.
The volatile hydrocarbon compounds can be further analyzed via gas chromatography (GC) and mass spectrometry (MS).
What is the order of acidity of hydrocarbons?
A pi bond is easier to break than a sigma bond; therefore, alkynes are generally more reactive than alkenes, followed by alkanes containing an equal number of C-atoms.
In accordance with that, the acidity (ability to lose an H+ ion) of hydrocarbons follows the order:
alkynes > alkenes > alkanes.
The concept of hyperconjugation/ no bond resonance in hydrocarbons
Hyperconjugation refers to the conjugation of C-H bonded electrons with the 𝛑 electrons of an unsaturated system such as a C=C or C≡C bond. Therefore, hyperconjugation is present in alkenes or alkynes.
Double and single covalent bonds present at adjacent positions in a molecule lead to partial delocalization of the otherwise localized sigma-bonded electrons. It is thus also sometimes known as sigma-pi (σ- 𝛑) resonance or no-bond resonance, as the C-H bond temporarily breaks inducing an ionic character in the hydrocarbon chain or ring.
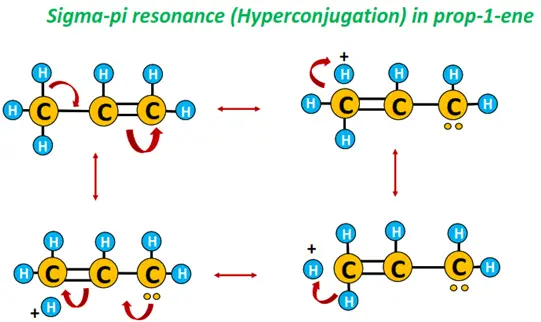
Example: Hyperconjugation in prop-1-ene.
As shown below, any of the three C-H bonds in the methyl (CH3) group of prop-1-ene can break at one time to yield three different canonical forms. The actual structure of prop-1-ene is a hybrid of all these canonical forms.
Why are hydrocarbons important? – Uses and applications
Although comprised of only 2 elements, there are millions of hydrocarbons naturally found and those synthesized by chemists in their labs.
- Hydrocarbons are found in plants, animals and fossil fuels.
- Natural gas is a hydrocarbon mixture comprised of methane (CH4) and ethane (C2H6).
- Hydrocarbons are primary components of natural reserves such as coal, crude oil, and petroleum.
- Petroleum refining gives us different lengths of valuable hydrocarbons for jet fuels, diesel, heating oil, LPG etc.
- Alkenes and alkynes are chief components of polymers such as plastic (high-density polyethylene, low-density polyethylene, polypropylene) used in food trays, milk bottles, shopping bags, etc.
- Aromatic hydrocarbons are used as solvents for manufacturing pesticides, paints and detergents.
Quiz yourself on organic hydrocarbons here.
Test your knowledge of the nomenclature of branched hydrocarbons here.
References
1. M. Younas. 2015. A textbook of Organic Chemistry.
2. Morrison, Robert Thornton, Robert Neilson Boyd, and Saibal Kanti Bhattacharjee. 2013. Organic Chemistry
3. Sparkman, O. David, Zelda E. Penton, and Fulton G. Kitson. 2011. ‘Chapter 21 – Hydrocarbons.’ in O. David Sparkman, Zelda E. Penton and Fulton G. Kitson (eds.), Gas Chromatography and Mass Spectrometry (Second Edition) (Academic Press: Amsterdam).
