Alkyl halides and aryl halides are substituted hydrocarbons in which one or more halogen (X) atoms are covalently bonded to the carbon atoms. These are used as building blocks in pharmaceuticals, agrochemicals, refrigerants, propellants, etc.
In this article, we will explore in detail the chemistry, structure, properties, reactions and applications of alkyl halides and aryl halides. So, continue reading!
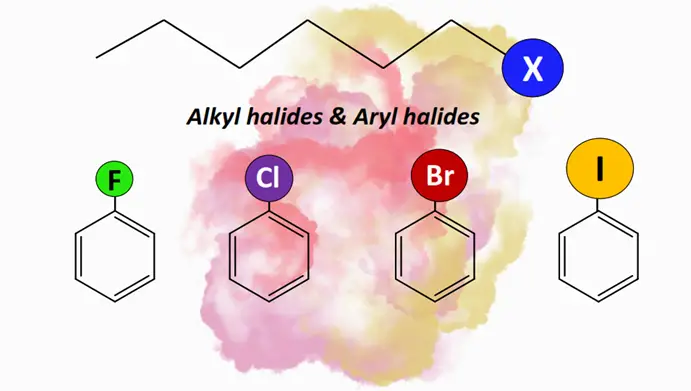
Alkyl halides: Definition, structure & nomenclature
Alkyl halides, or halogenoalkanes (R-X), are organic compounds in which a halogen (X) atom is attached to an alkyl (R) group. The general formula for an alkyl halide is CnH2n+1 X. The halogen atoms such as fluorine (F), chlorine (Cl), bromine (Br) and iodine (I) belong to Group VII A (or 17) of the Periodic Table of Elements. The R group in alkyl halides may contain a single C-atom or be a long hydrocarbon chain.
For example, Chloromethane (CH3Cl) is a simple alkyl halide in which the Cl-atom is attached to a methyl (CH3) group.

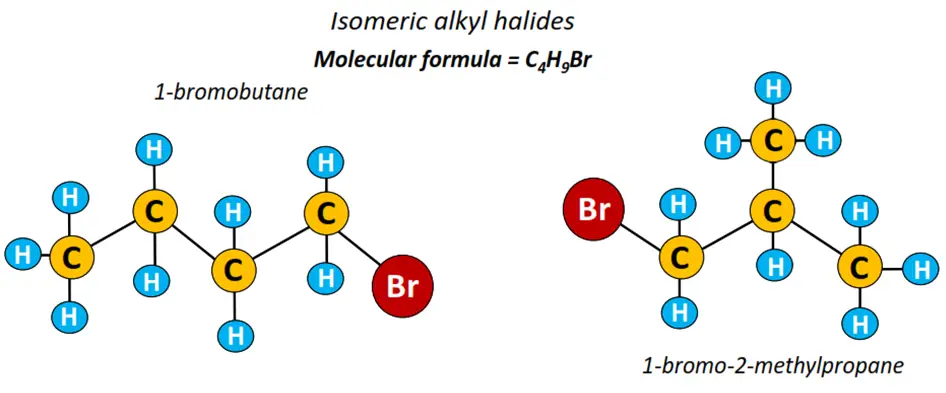
1-bromobutane or n-butyl bromide is a long-chain alkyl halide represented by the structure CH3-CH2-CH2-CH2-Br. As the halogen atom is attached to carbon no. 1 in the alkyl chain containing 4 C-atoms, it is thus called 1-bromobutane. This implies that alkyl halides can form both straight chain structures as well as their branched isomers.

1-bromo-2-methylpropane is a branched isomer of 1-bromobutane; their structures shown below.
An alkyl halide is named following the pattern given below.
Carbon no. at which the halogen atom is present – halogen name (halo)- carbon no. carrying an extra alkyl group – name of alkyl group – name of the long hydrocarbon chain.
Example: To name the alkyl halide structure shown below:
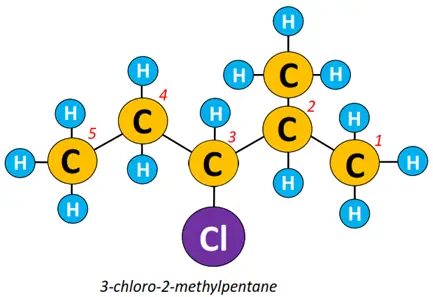
- The carbon chain is numbered, starting from the terminal closest to an extra R-group.
- The halogen atom, which is a chlorine atom, is attached at C-3, so it is 3-chloro.
- A methyl (CH3) group is attached at C-2 so it is 3-chloro-2-methyl.
- The long hydrocarbon chain contains a total of 5 C-atoms, representing a pentane molecule.
So the name of the above structure is 3-chloro-2-methylpentane.
The alkyl halides can also be classified into primary, secondary and tertiary halogenoalkanes.
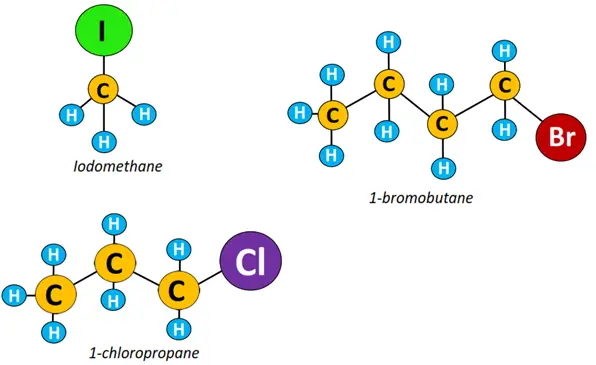
- Primary (1°) halogenoalkane: In a primary alkyl halide, the halogen atom is attached to a carbon linked to 1 other C-atom only. Examples include 1-bromobutane, 1-chloropropane, and iodomethane.
- Secondary (2°) halogenoalkane: In a secondary alkyl halide, the halogen atom is attached to a carbon having 2 C-atoms at the sides. Examples include 2-chloropropane (also called isopropyl chloride) and 3-chloro-2-methylpentane.

- Tertiary (3°) halogenoalkane: In a tertiary alkyl halide, the carbon atom carrying the halogen is attached to 3 other C-atoms, such as 3-bromo-3,5-dimethylhexane.

Properties of alkyl halides
Alkyl halides are:
- Typically, colorless, odorless liquids or solids at room temperature having higher melting and boiling points than their parent alkane molecules due to an additional C-X bond. For a given alkyl group, the boiling point of the alkyl halide increases with the increase in atomic weight of the halogen attached. In contrast, branching lowers their boiling points.
- Slightly polar due to a polar C-X bond.
- Generally insoluble in water but soluble in organic solvents such as ethers and ketones.
- Stable. The stability of an alkyl halide increases with increasing size and decreasing electronegativity of the halogen atom.
- Toxic in nature.
Preparation of alkyl halides
1.From alkenes
The acid-catalyzed electrophilic addition of alkenes forms alkyl halides.
Reagent: Hydrogen halides (HX) such as HCl, HBr, HF, etc.
Mechanism: The C=C double bond breaks, an H-atom is added on one C-atom and the halogen (X) atom is added to the adjacent carbon.
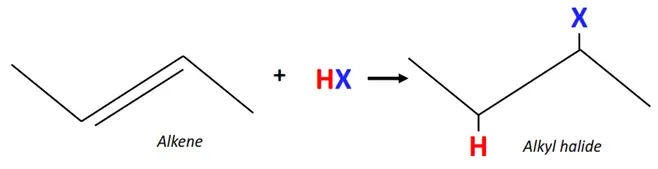
The addition reaction of alkenes to form alkyl halides follows the Markovnikov rule.
Markovnikov’s rule states that when a protic acid (HX) is added to an asymmetric alkene, the H-atom is added to a carbon already carrying a greater number of hydrogen substituents.
For instance, in the reaction shown below, the H-atom is preferably added to C-1 and not C-2.

Logic behind Markovnikov’s rule: A carbocation intermediate is formed during an electrophilic addition reaction. A tertiary carbocation is more stable than a secondary carbocation, followed by the stability of a primary carbocation. The more stable the carbocation, the greater its chances of transforming into the final product.

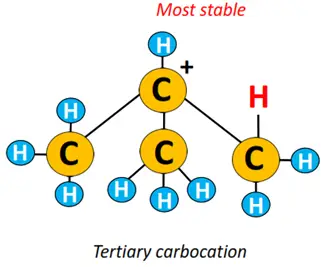
Therefore, product-1 is major, while product-2 is retrieved as a minor product from the above reaction.
2. From alcohols
The nucleophilic substitution of alcohols gives alkyl halides.
Different reagents can be used for this reaction, giving different by-products.
- Conc. HX or NaX, H2SO4 and heat.
- PCl3, PCl5, SOCl2 to prepare chloroalkanes.
- PBr3 to prepare a bromoalkane.
A catalyst is required in this reaction for primary and secondary alcohols however, no catalyst is needed for the nucleophilic substitution of tertiary alcohols.
Mechanism: The OH functional group of an alcohol is replaced with the halogen atom.
This is the most widely used method for alkyl halide preparation. A primary halogenoalkane is prepared from primary alcohol, secondary halogenoalkane from secondary alcohol, and tertiary halogenoalkane from substituting a tertiary alcohol.

3. From hydrocarbons
The free radical substitution of certain hydrocarbons in the presence of UV light or heat gives halogenoalkanes.

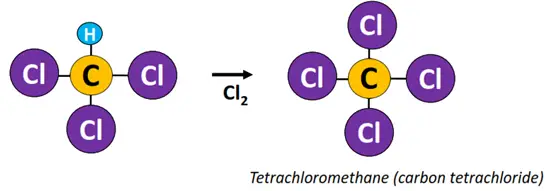
Example: Free radical substitution of methane (CH4).
Reagent & Conditions: Cl2, heat or light
Mechanism:
- Initiation: The Cl-Cl bond undergoes homolytic fission in the presence of high-energy UV radiations to produce Cl• free radicals.

- Propagation: A C-H bond in methane undergoes heterolytic fission to produce •CH3 free radical.
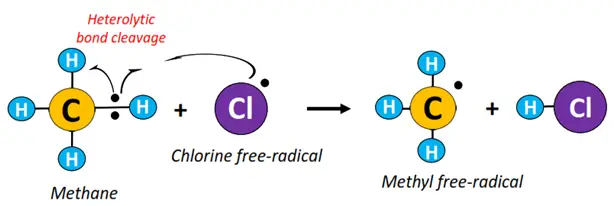
- Termination: •CH3 combines with Cl• to produce CH3Cl (methyl chloride).
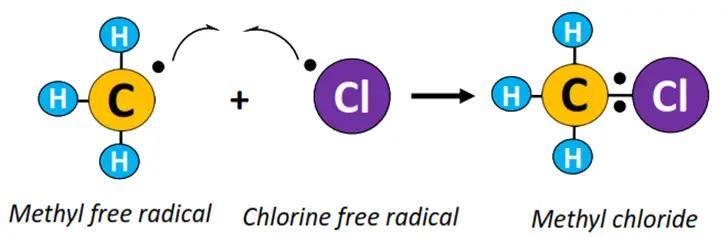
The above reaction continues to form other haloalkane derivatives, such as dichloromethane (CH2Cl2), trichloromethane (CHCl3), also known as chloroform, until all the H-atoms in the reaction mixture are replaced with Cl-atoms and tetrachloromethane (CCl4) is obtained as an end product.

Reactions of alkyl halides
Alkyl halides generally react via nucleophilic substitution and elimination reactions.
Nucleophilic substitution reactions of alkyl halides
In the nucleophilic substitution reaction of halogenoalkanes, the halogen atom is replaced by another functional group, such as OH, CN, OR, etc.
This type of substitution, in turn, follows two different mechanisms:
- SN-1 mechanism
- SN-2 mechanism
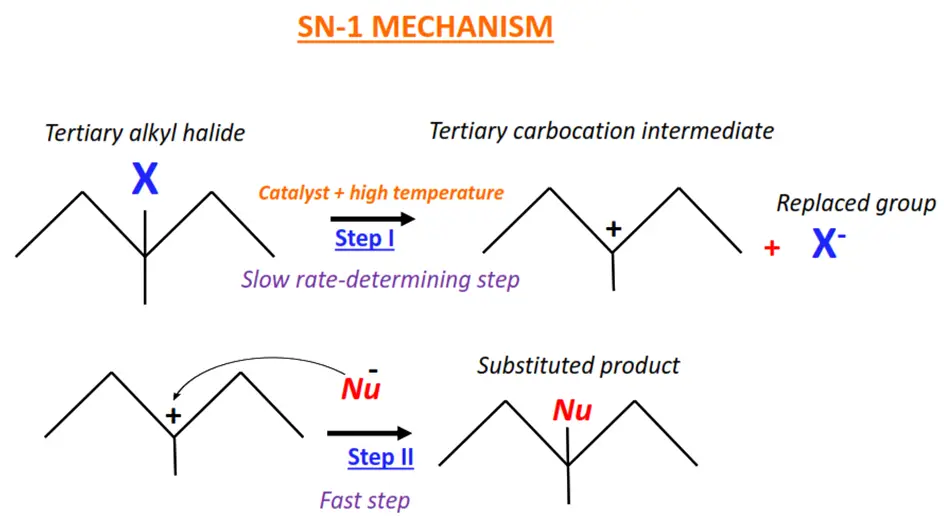
Tertiary halogenoalkanes typically undergo nucleophilic substitution by the SN-1 mechanism.
The SN-1 mechanism follows first-order kinetics. It is a two-step, unimolecular mechanism as only 1 molecule is present at the slow rate-determining step of the reaction.
In the SN-1 mechanism, the C-X bond breaks in the presence of a catalyst and high temperature.
A carbocation intermediate is formed, which is then readily attacked by the nucleophile (Nu) to give the final product.
In comparison, primary and secondary halogenoalkanes react via the SN-2 mechanism.
The SN-2 mechanism of nucleophilic substitution follows second-order kinetics. It is a bimolecular single-step reaction in which a total of 2 molecules are present at the slow rate-determining step. Unlike the SN-1 mechanism, there is no intermediate in the SN-2 reaction; contrarily, it involves a transition state.
The nucleophile (Nu) possessing a lone pair of electrons attacks Cδ+ of the alkyl halide, opposite from the halogen atom. No bulky alkyl groups present in primary alkyl halides means a negligible hindrance to the temporary attachment of a fifth group in the transition state. The C-X bond then breaks, stabilizing the new C-Nu bond.

In the SN-2 mechanism, the configuration of the final product is usually inverted w.r.t the parent halogenoalkane. This process is called Walden Inversion.
Particular examples of nucleophilic substitution of alkyl halides are:
- Alcohol synthesis
- Williamson ether synthesis
- Ester synthesis
- Nitrile synthesis
- Amine synthesis

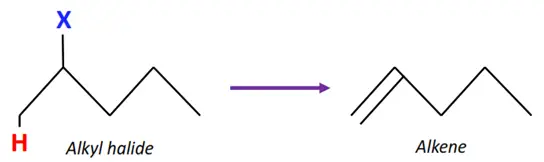
Elimination reactions of alkyl halides
The elimination of the halogen atom from one C-atom and hydrogen from the adjacent C-atom forms a C=C double bond, i.e., an alkene molecule.
Preparation of Grignard’s reagent from alkyl halides
Alkyl magnesium halide (R-Mg-X), an organometallic compound, is formed by the chemical reaction of an alkyl halide with magnesium metal in the presence of dry ether.

Reduction reaction of alkyl halides
Alkyl halides are reduced to corresponding alkane molecules using alkali metals (Li, Na, K) and acids (H+) as reducing agents.

Alkyl halide uses and applications
Alkyl halides are used:
- As solvents in organic synthesis, such as chloroform (CHCl3) and carbon tetrachloride (CCl4).
- As starting materials and alkylating agents in the preparation of drugs, pharmaceuticals and biologically active compounds. Ethyl chloride is used as a local anesthetic in minor surgeries.
- In producing refrigerants, propellants, air conditioning systems, aerosol sprays, etc. However, you may note that the modern scientific world prohibits using chlorofluorocarbons (CFCs) as coolants and in aerosol sprays due to their ozone-depleting properties.
- As flame retardants. Brominated flame retardants are used to make plastics and other materials resistant to fire.
Aryl halides: Definition, structure & chemistry
Aryl halides (Ar-X), also known as haloarenes, are organic compounds in which the halogen (X) atom is directly attached to an aromatic ring, such as the phenyl (C6H5) ring.
Halogen atoms attached to the phenyl ring act as ring deactivators. The halogens deactivate the phenyl ring by an electron-withdrawing inductive effect (I-).
An X-atom is more electronegative than its adjacent C-atom. So, it largely attracts the C-X electron cloud towards itself. The delocalized electron cloud decreases within the ring. Therefore, the reactivity of the ring decreases towards electrophilic substitution reactions.
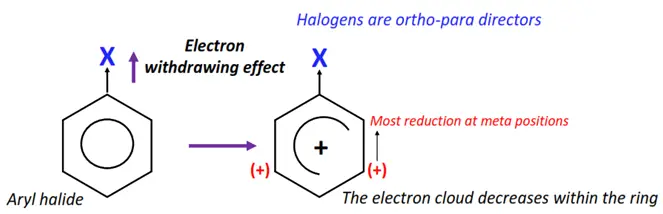
However, halogens are an exception of the deactivating group as they are ortho-para directing.
An example of an aryl halide is chlorobenzene. Chlorobenzene undergoes electrophilic substitution at a slower rate as compared to benzene. p-nitro chlorobenzene is obtained as the major product via chlorobenzene nitration.

Preparation of aryl halides
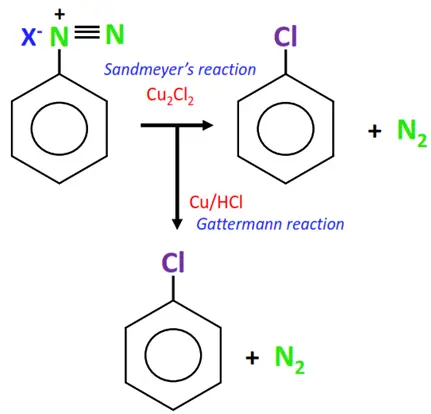
1. From diazonium salts
The replacement of nitrogen from a diazonium salt produces an aryl halide.
Diazonium salts (R-N2+) are highly reactive organic compounds with a positively charged N-atom directly attached to the aromatic ring.
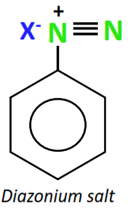

Mixing a freshly prepared solution of the diazonium salt with cuprous chloride (CuCl) or cuprous bromide (CuBr) results in the replacement of the diazonium group with the halogen atom, thus producing an aryl halide. This chemical reaction is known as Sandmeyer’s reaction.
In a modified version of Sandmeyer’s reaction, the relatively less stable and difficult-to-handle cuprous halides are replaced with copper powder and hydrogen halide as reagents. This is known as the Gattermann reaction.
2. From benzene
The direct halogenation of a benzene ring in the presence of a Lewis acid and the halogen results in an electrophilic substitution reaction. It produces the required haloarene, while hydrogen halide is released as a by-product.

However, this method is rarely used as it gives a mixture of ortho, para, and meta products, unlike specific aryl halide synthesis, as expected in Sandmeyer’s reaction.
Reactions of aryl halides
- Electrophilic aromatic substitutions of aryl halides.
- Nucleophilic substitutions of aryl halides.
Aryl halides undergo nucleophilic substitutions only if the aromatic ring contains, in addition to halogen, certain other groups located ortho or para to the halogen.
Why do aryl halides react with Grignard’s reagents?
Similar to alkyl halides, aryl halides react with magnesium metal to form aryl magnesium halide (Grignard reagent) in dry ether.
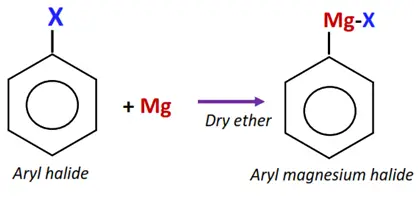
The Grignard reagent, in turn, can react with aryl halides to drive a series of chemical reactions, introducing the aryl group into various other organic compounds.
The C-atom in the Grignard reagent acts as a nucleophile and attacks the aryl halide, displacing the halogen atom and forming a new C-C bond.
Aryl halide uses and applications
Aryl halides are used in:
- Organic synthesis, such as using aryl halides as substrates in cross-coupling reactions, produces other biaryl complex molecules.
- Agrochemical (pesticides, fungicides, insecticides) synthesis. For instance, 2,4-dichloro phenoxy acetic acid (2,4-D) is an aryl halide derivative used to control broadleaf weeds in agricultural crops.
- Polymerization to prepare different varieties of plastics, coatings and fibers.
Differences between alkyl halides and aryl halides
| Alkyl halides (Halogenoalkanes) | Aryl halides (Haloarenes) |
| The halogen atom is attached to a hydrocarbon chain | The halogen atom is attached to an aromatic ring |
| Derived from open-chain alkanes | Derived from cyclic, ringed structures |
| The C-X bonded carbon is sp3 hybridized | The C-X bonded carbon is sp2 hybridized |
| No delocalized electrons | Pi-bonded delocalized electrons present within the ring |
| Generally more reactive than aryl halides | Generally, less reactive than alkyl halides due to a strong C-X bond |
| Usually undergo a nucleophilic substitution reaction. | Usually undergo an electrophilic substitution reaction. |
For additional reference, practice your concepts on alkyl halides and aryl halides here.
Also, test yourself using the Q&A worksheet attached here.
References
1. H.Brown, W. & POON, T. 2016. Introduction to Organic Chemistry.
2. Morrison, R. T., Boyd, R. N. & Bhattacharjee, S. K. 2013. Organic Chemistry.
3. Ouellette, R. J. & Rawn, J. D. 2018. 9 – Haloalkanes and Alcohols Nucleophilic Substitution and Elimination Reactions. In: OUELLETTE, R. J. & RAWN, J. D. (eds.) Organic Chemistry (Second Edition). Academic Press.
