Mastering organic chemistry takes time and practice. It might seem a daunting task at once if the right approach is not adopted.
But in our organic chemistry series, we have tried to break down all the important information about organic chemistry into brief and comprehensive articles.
So in this final article, we have summed up all the basics along with providing you with some quirky tips and tricks which you can use to ace your organic chemistry exam.
You may also like to check out the organic chemistry reactions and mechanisms cheat sheets provided at the end of the document. But, for all that, you have to continue reading till the end!
8 Tips & Tricks to study organic chemistry
I) Break down the insurmountable information into small, easily digestible chunks
There are numerous organic families based on the different functional groups attached, such as:
- Hydrocarbons
- Alcohols
- Carboxylic acids
- Alkyl halides
- Carbonyl compounds, etc.
Dealing with one functional group and its family at a time, learning its chemistry, preparation, reactions and uses keeps the information easily digestible. So study one organic chemistry chapter at a time and make notes on the key concepts.
II) Learn the language
Language is the foundation stone for communication. Thus, learning organic nomenclature is very important to convey the structure, composition and properties of organic compounds.
To study organic nomenclature, you may learn:
- The IUPAC rules and conventions for identifying and naming organic compounds
- To name the parent carbon chain
- To identify and prioritize the substituents attached to the parent chain
- To use mnemonics for remembering common prefixes and suffixes.
- To practice with examples
III) Understand the concepts instead of memorizing text
Organic chemistry is largely a conceptual science. So, having a firm grip on concepts such as atomic structure, molecular geometry, functional groups, conjugation, resonance, isomerism, etc., is vitally important before proceeding to advanced topics and reaction mechanisms.

IV) Practice makes a man perfect
Given above is an ages-old saying, often considered a cliché. But it’s actually not because working through practice problems, multiple choice questions (MCQs), and quizzes on the different sections of organic chemistry definitely helps you build concepts.
V) Draw organic structures and reaction mechanisms by yourself
It is a must that if you want to learn organic structures and mechanisms, you need to draw them.
There is no shortcut for that, except that you can draw these structures using paper and a pencil or with the aid of advanced computer softwares such as ChemDraw and ChemSketch.
Drawing, first looking through the Book and then by your heart, speeds up the learning process.
VI) Visualize molecular changes by identifying trends and patterns.
Every chemical change takes place for a reason and follows a specific trajectory. Therefore, you can look for similarities and differences when studying organic chemistry reactions. This will help you make connections between different functional groups and key principles.
For instance, alcohols are produced by reducing aldehydes and ketones, while alcoholic oxidation yields carboxylic acids. Conversely, a carboxylic acid and an alcohol react to form an ester.
You can visualize these functional group transformations using molecular model kits, writing balanced chemical equations, and studying energy profile diagrams.
VII) Get rid of all confusion.
One misconception can lead to the other and then another while studying organic chemistry. Therefore, getting clear, profound answers to all the questions that fog your mind is integral.
However, the good news is that plenty of academic support is available online. You can access the web and search for all organic chemistry-related content in the Chemistry section of easy to calculate.com.
Additionally, you can search for educational videos on YouTube and, of course, go for the conventional method, i.e., jot down your queries and ask your professors for help.
Moreover, forming study groups with like-minded friends and classmates is a valid option for clearing confusion.

VIII) Trust the process with a positive outlook
Lastly, studying organic chemistry is a process, not a one-day task. So, try keeping it in your practice daily to save yourself from last-minute exam anxiety and nervousness. A solid background in organic chemistry will help you stay in touch with modern scientific research and understanding, and you will begin to appreciate life better.
So, we encourage you to keep a positive outlook as you dive into the fascinating world of organic compounds.
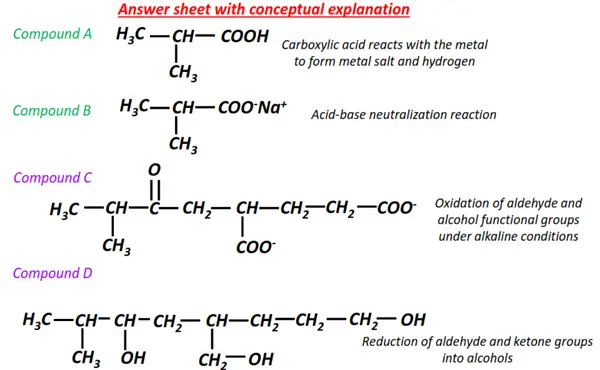
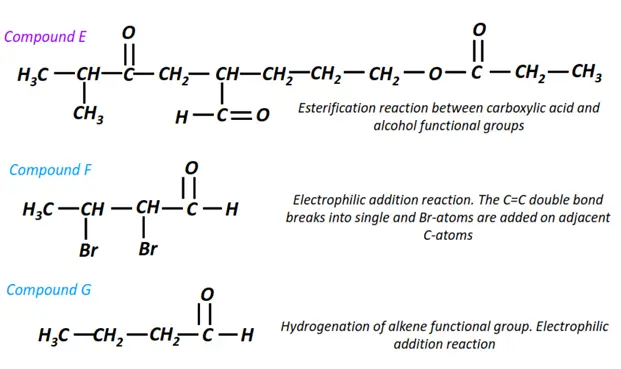
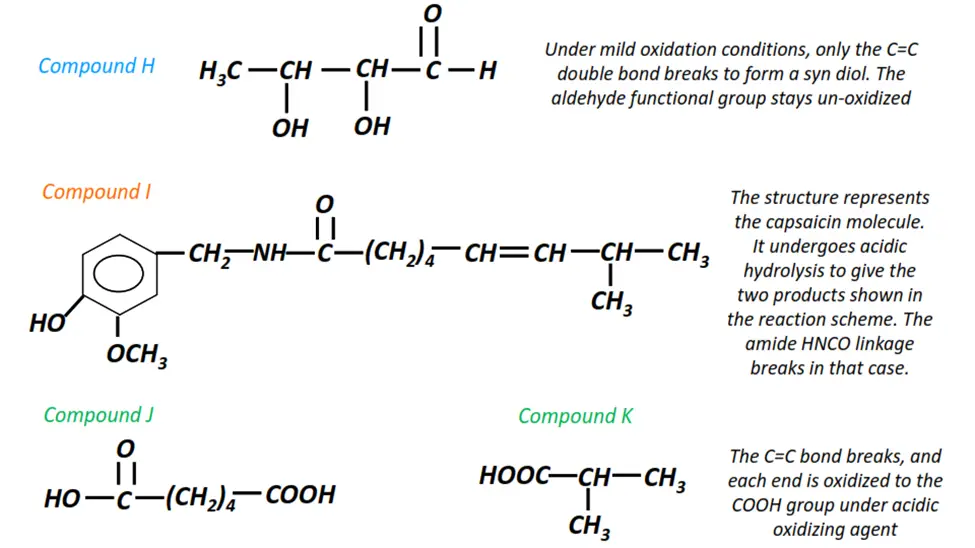
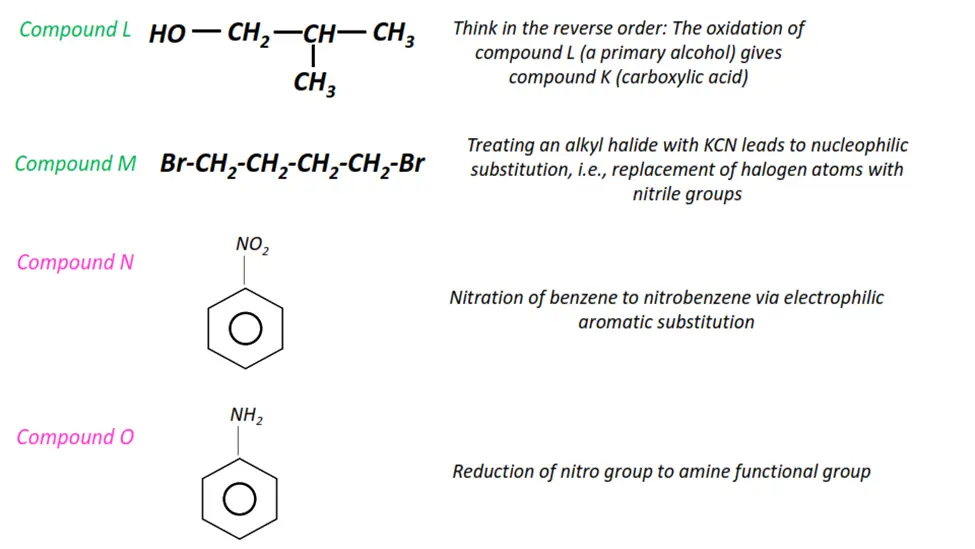
Organic chemistry- reactions & mechanisms- Cheat sheets & practice questions
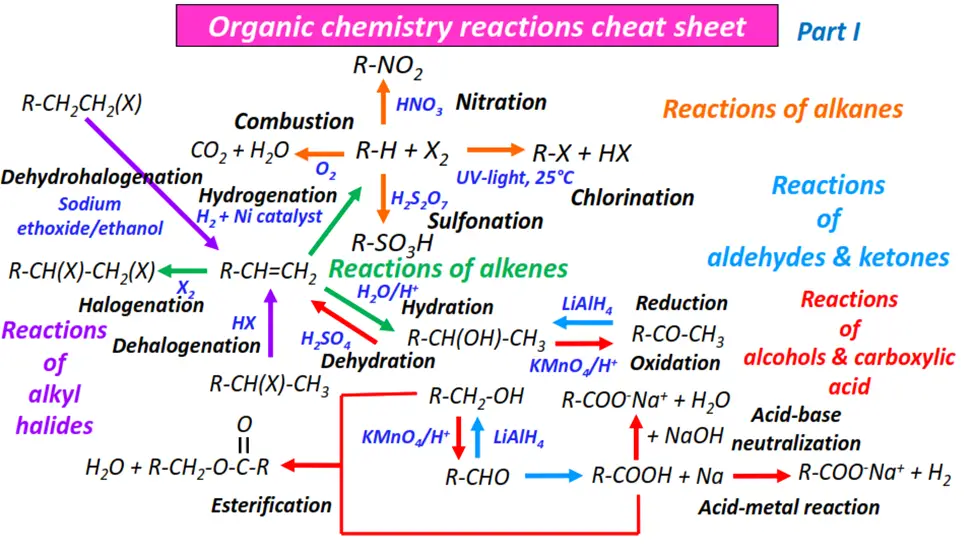
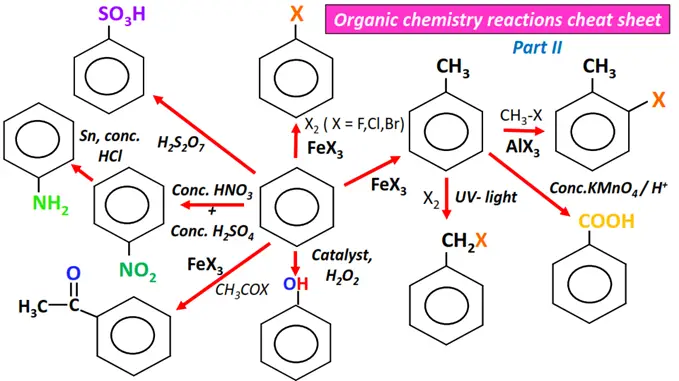
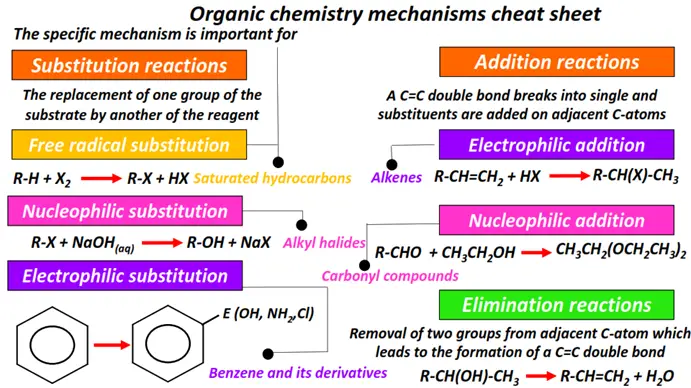
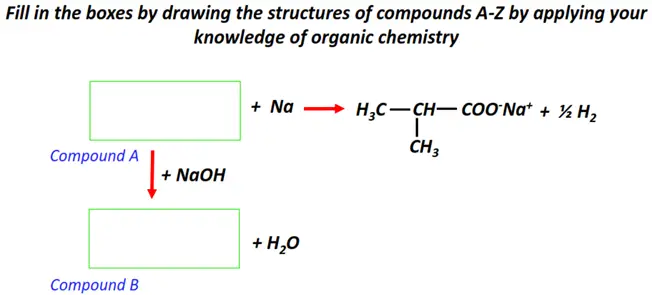
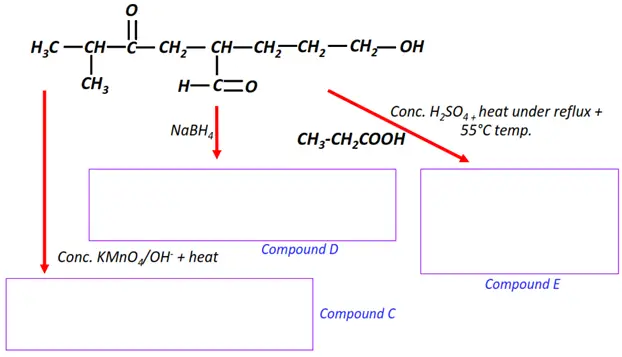
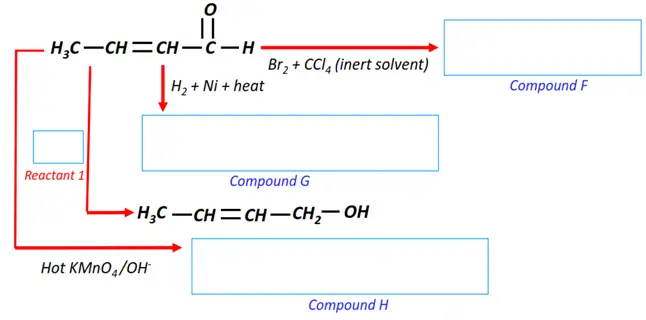
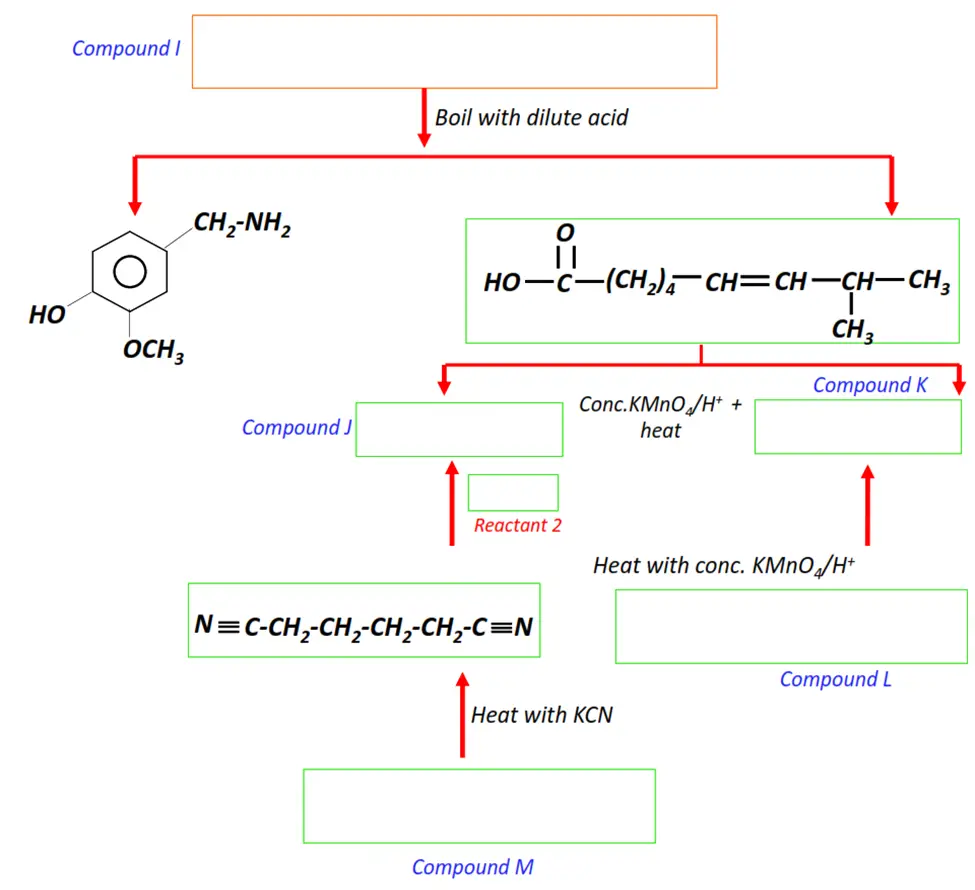
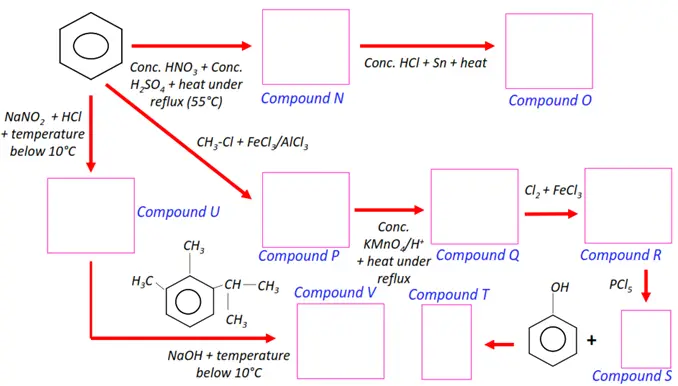
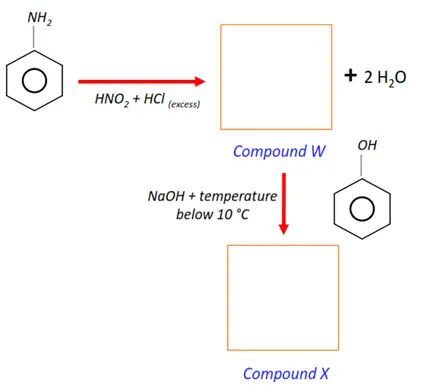
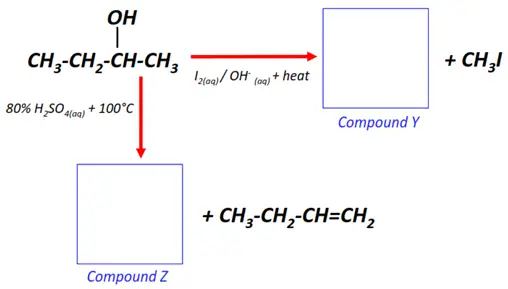
Note: You will find the answers to the above problems at the end of this article.
Organic chemistry key concepts- Summary
- Organic chemistry is the study of carbon compounds.
- Carbon is a unique element as it can self-attach to form long carbon-carbon chains and rings of all sizes. This unique self-linking property of carbon is called catenation.
- Hydrocarbons are organic compounds containing carbon (C) and hydrogen (H) atoms only.
- Organic hydrocarbons are divided into saturated and unsaturated hydrocarbons. Alkanes are an example of saturated hydrocarbons, while unsaturated hydrocarbons include alkenes and alkynes.
- Aromatic hydrocarbons are cyclic unsaturated molecules. These possess planar, ringed structures and follow Huckle’s (4 n + 2)𝛑 electrons rule.
- Benzene (C6H6) is the parent member of the aromatic family. It consists of 3 C-C single and 3 C=C double covalent bonds at alternating positions. Thus, it is known as a conjugated molecule.
- The pi-bonded electrons present in benzene keep revolving from one position to another on the molecule in order to decrease its energy. However, increasing its overall stability.
- The delocalization of pi-bonded electrons and unshared electrons in an organic molecule is known as resonance.
- A resonance hybrid is a weighted average of all the possible resonance structures (canonical forms) of an organic molecule.
- Resonance-stabilized molecules are particularly more stable than those without any resonance present in them.
- C-H bonds exhibit sigma bond resonance, aka hyperconjugation.
- Alkyl groups (R) directly bonded to an unsaturated system possess hyperconjugation. A C-H sigma bond breaks temporarily, increasing electronic delocalization and, thus, overall molecular stability.
- The greater the alkyl substituents attached to a carbocation, the higher its stability.
- A carbocation is a cation containing a positively charged C-atom. These are usually formed as intermediates during an organic reaction.
- The stability of a carbocation increase in the order: Primary carbocation < Secondary carbocation < Tertiary carbocation.
- Alcohols contain a hydroxyl (OH) functional group. These are slightly acidic in nature due to their ability to donate a proton (H+).
- Alcohols are subdivided into primary, secondary and tertiary alcohols depending upon the number of alkyl chains attached to the C-atom carrying an OH functional moiety.
- Primary, secondary and tertiary alcohols can be distinguished from one another via the Lucas test.
- Ethers contain C-O-C linkage. The oxygen atom is bridged between two alkyl chains of the same or varying chain lengths in an ether molecule.
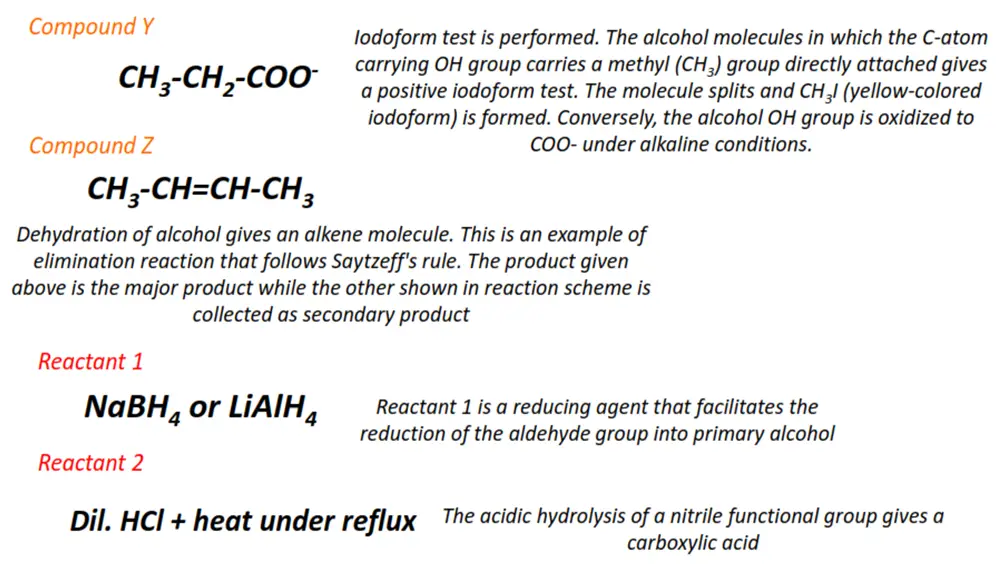
- The complete oxidation of a primary alcohol produces a carboxylic acid, while a secondary alcohol gets oxidized to a ketone.
- The incomplete oxidation of a primary alcohol under controlled (reflux) conditions produces an aldehyde.
- Aldehyde and ketones are carbonyl compounds comprising a C=O group.
- The 5 main types of reaction mechanisms in organic chemistry are free radical substitution, electrophilic addition, nucleophilic addition, nucleophilic substitution and elimination reactions.
- Carbonyl compounds undergo nucleophilic addition reactions, while electrophilic addition reactions are characteristic of alkene molecules.
- Carboxylic acids are organic molecules comprising one or more COOH functional groups.
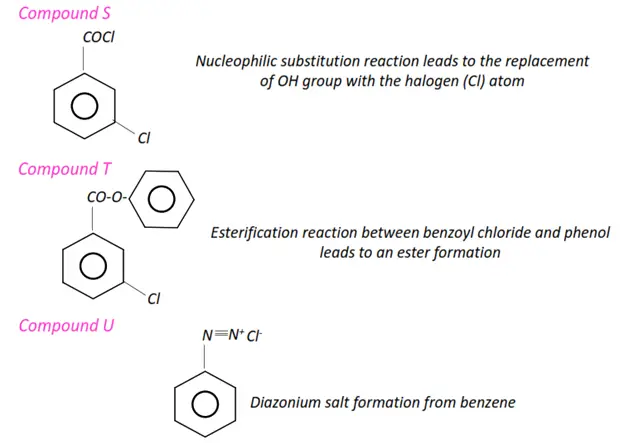
- The condensation reaction of a carboxylic acid with an alcohol gives an ester. This process is called esterification. The reverse of esterification is called hydrolysis.
- Phenols (Ar-OH) are more acidic than alcohols, as the phenolate ion (Ar-O–) is resonance stabilized. However, these are less acidic than carboxylic acid molecules.
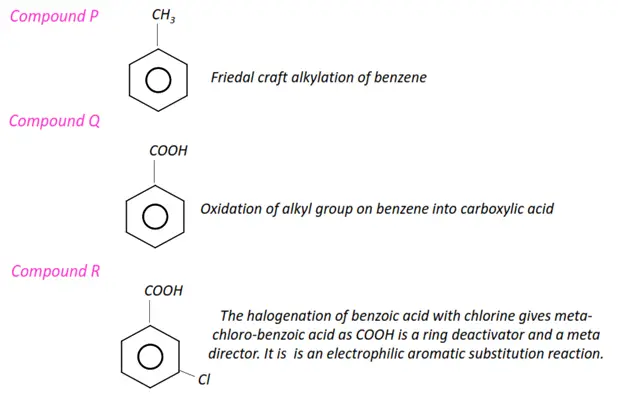
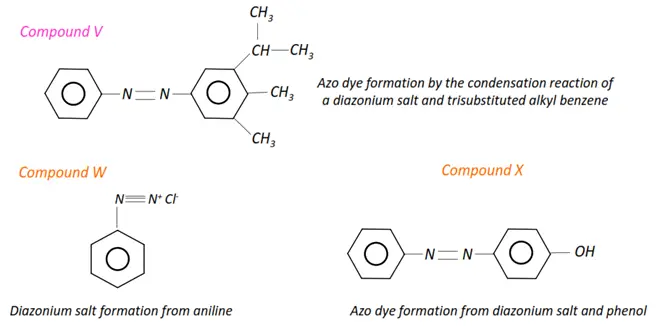
- Aromatic compounds such as benzene, aniline and phenols undergo electrophilic substitution reactions.
- Nucleophilic substitution reactions are characteristic of alkyl halides in which halogens (F2, Cl2, Br2) are directly attached to a carbon chain.
- In aryl halides, the halogen atoms are directly bonded to the phenyl rings.
You may also like our articles: