The importance of organic chemistry is unparalleled. Modern-day chemists say that we live in the Age of Carbon, and the study of carbon compounds is called organic chemistry.
Previously, organic compounds were perceived as chemical compounds derived from natural animal and plant sources only, such as fossil fuels and petroleum. Nevertheless, advanced scientific research proves that organic compounds can be natural as well as synthetic in origin.
However, the one thing that is common among all organic compounds is the element carbon (symbol C).
Let us explore what is carbon and why it is important before we discuss everything you need to know about organic chemistry in this article.
Carbon- a brief introduction
Carbon (C) belongs to Group 14 of the Periodic Table. Its electronic configuration is 1s2 2s2 2p2. The valency of carbon is thus four, which means a carbon atom can form a total of 4 covalent bonds.
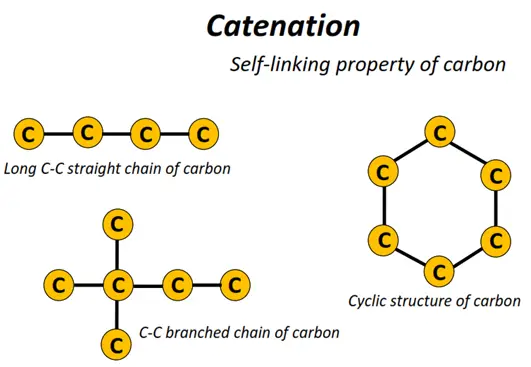
Carbon is a versatile element because C-atoms can get strongly attached to one another, unlike any other elemental atom. Thousands of interlinked carbon atoms can form chains and rings of all sizes. This unique property of carbon is known as ‘catenation’’.
The rings and chains formed comprise C-C single, double or triple covalent bonds and can have crosslinks or branches, respectively.
In addition to that, plenty of other atoms, such as hydrogen (H), oxygen (O), nitrogen (N), sulfur (S), halogens, etc., can get attached to long C-C chains and form a variety of new chemical compounds called organic compounds.
All the different organic compounds formed possess unique physicochemical properties, which means they differ in appearance, color, smell, melting and boiling point, reactivity, etc. That is why organic chemistry is a whole new field of science that we need to study in detail.
What is organic chemistry? – History and origins
Organic chemistry had its beginning back in the 16th century when organic compounds were isolated from natural resources in their pure state. Later on, the Vital force theory was presented by Berzelius in 1809. In his lab, Friedrick Wohler, a German scientist, rejected this concept by synthesizing urea, an organic compound. However, it was in the 1950s that amino acids were synthesized for the first time by lab experiments incorporating carbon dioxide, methane, nitrogen and ammonia.
Definition
Organic chemistry is the study of carbon-containing compounds, including their chemical composition, structure, formation, physicochemical properties, reactivity, etc.
Basics and fundamentals of organic chemistry
The physicochemical properties of an organic compound directly depend on its structure which in turn depends on the constituent atoms, bond connectivity, orientation in space, etc. So the structure and bonding of compounds lie at the heart of organic chemistry.
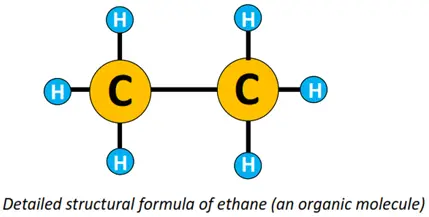
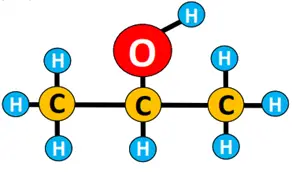
An organic compound or molecule is thus represented by its structural formula. For instance, the molecular formula for ethane is C2H6. Its condensed structural formula is CH3-CH3, while its detailed structural formula is shown below.
Similarly, both the detailed and simplified structural formulae for n-butane are given below.
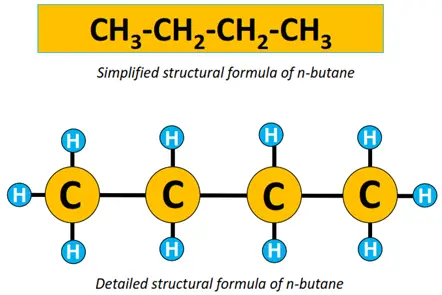
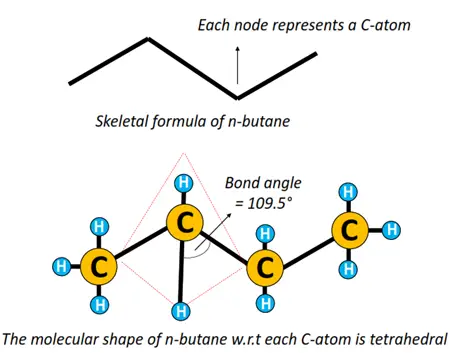
A single straight line in between atoms represents a single covalent bond. The prefix n denotes that it is a straight chain structure. n-butane can also be represented by a zigzag representation, omitting elemental symbols. Each C-C-C bond angle = 109.5° as the shape of the molecule w.r.t each C-atom is tetrahedral.
The most important organic chemistry concepts
In organic compounds, C-C single bond is always a sigma (σ) bond. In the C=C double bond, the first bond is always a sigma bond, while the second is a pi (𝛑) bond.
If a carbon atom is bonded to 2 other C-atoms, it must then have 2 H-atoms to complete its valency of 4 and attain a stable octet electronic configuration.
Organic compounds are primarily classified into:
- Hydrocarbons: Hydrocarbons are compounds composed of carbon and hydrogen atoms, containing C-C and C-H bonds only.
Hydrocarbons are subdivided into the following homologous series:
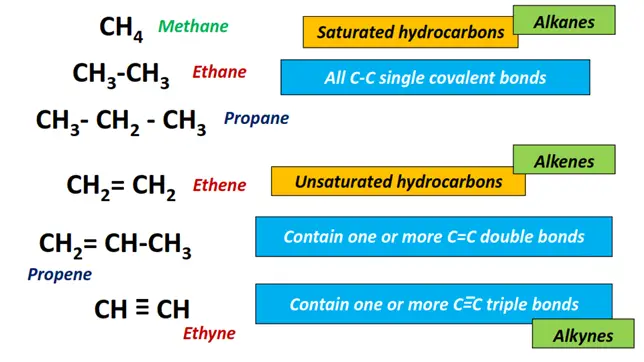
- Alkanes: An alkane or paraffin is an acyclic saturated hydrocarbon with the general formula CnH2n+2 where n= number of carbon atoms. It consists of all C-C single bonds in the long chain. Examples include methane (CH4), ethane (C2H6), propane (C3H8), etc.
- Alkenes: Alkenes, informally known as olefins, are unsaturated hydrocarbons having a general formula of CnH2n. It consists of C-C single as well as C=C double bonds within the straight chain as well as branches can be formed.
Examples include ethene (C2H4), propene (C3H6), butene (C4H8), etc.
n-butene represents a straight chain structure of butene while 2-methyl-prop-1-ene is a branched isomer of n-butene.
Similarly, n-butene can be represented as but-1-ene or but-2-ene, where but-1-ene and but-2-ene are positional isomers that differ depending upon the position of the C=C double bond.
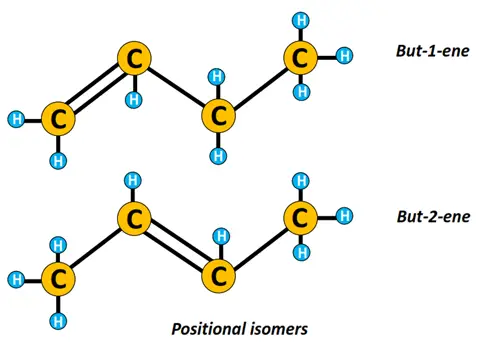
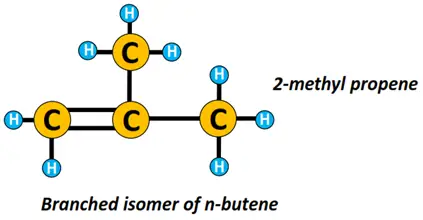
Isomerism is another important concept in organic chemistry. Isomers are chemical compounds having the same molecular formula but a different structural arrangement.
3. Alkynes: An alkyne is also an unsaturated hydrocarbon. It consists of C ≡ C triple covalent bonds in the carbon chain. The homologous series is represented by a general formula CnH2n-2. Acetylene or ethyne (C2H2) is the simplest alkyne molecule. Other examples include propylene (C3H6), butyne (C4H8), etc.
An H-atom removed from a hydrocarbon yield a radical (highly reactive specie), i.e., an alkyl group. The alkyl (-R) group, such as methyl (-CH3) or ethyl (CH3CH2-), has an incomplete valency, so it can readily bind with another functional group to form a new organic compound.
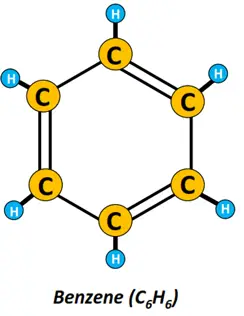
- Aromatic hydrocarbons: Aromatic hydrocarbons are cyclic unsaturated organic compounds that consist of one or more planar rings composed of carbon and hydrogen atoms. Benzene (C6H6) is undoubtedly the most important member of the aromatic family.
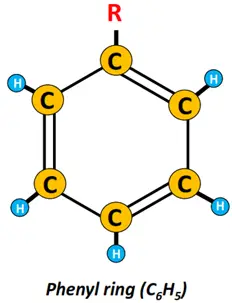
Removing an H-atom from the benzene ring produces a highly reactive phenyl (C6H5) group.
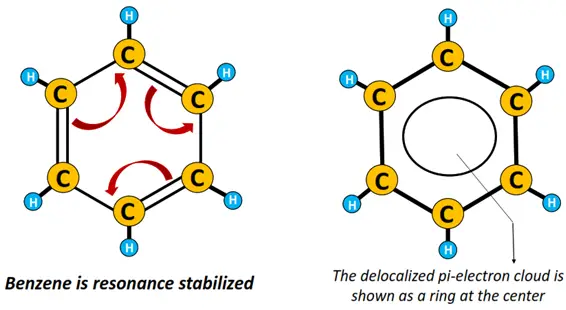
Tightly held, C-C and C-H sigma-bonded electrons are referred to as localized electrons. Contrarily, pi-bonded electrons or electrons present as lone pairs on an atom are delocalized.
A large number of delocalized electrons present in the hexagonal benzene structure are represented as a ring at the center of the molecule.
The benzene ring is resonance stabilized.
Resonance is another important concept in organic chemistry. It simply refers to the movement of delocalized electrons in a conjugated organic molecule which is a molecule containing alternately arranged C-C single and C=C double covalent bonds.
Resonance is only applicable to unsaturated systems. However, saturated hydrocarbons exhibit sigma bond resonance (or hyperconjugation).
Different functional groups in organic chemistry
Millions of organic compounds on Earth are formed, containing many different functional groups. These organic functional groups include the following:
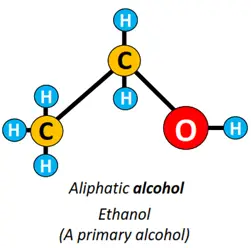
- Hydroxyl (-OH) functional group: If an OH group is attached to an alkyl group, the organic compound formed is known as alcohol. Methanol (CH3OH) and ethanol (CH3CH2OH) are examples of alcohol.
Alcohols are subdivided into primary, secondary and tertiary alcohols depending upon the placement of the OH group. Both CH3OH and CH3CH2OH are primary alcohols, while CH3CH(OH)CH3 is a secondary alcohol.
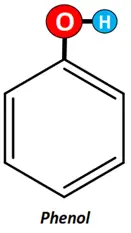
If the OH group is attached to the phenyl ring, it is known as phenol.
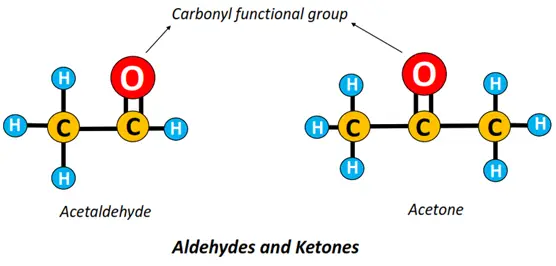
- Carbonyl (-C=O) functional group: If C=O is attached to an alkyl group; the resultant compound is called a carbonyl compound.
There are 2 main types of carbonyl compounds:
- Aldehydes (-R-CHO): An aldehyde is an organic compound in which a C-atom is double-bonded to an O-atom on one side and a hydrogen (H) atom on the other. Acetaldehyde is the simplest aldehyde with the formula CH3CHO.
- Ketones (-R-CO-R): In ketones, the carbon-carrying double-bonded O-atom is bonded to two other C-atoms or alkyl chains at the sides.
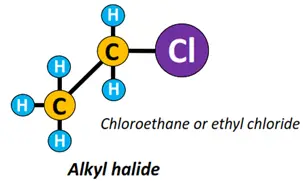
- Halide (X) functional group: An alkyl halide or halogenoalkane (R-X) is formed if a halogen (X) atom such as chlorine (Cl), fluorine (F), bromine (Br), etc. are, attached to an alkyl chain.
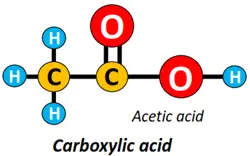
- Carboxyl (-COOH) functional group: The carboxylic acid (R-COOH) is an organic compound containing a carboxyl functional group attached to the alkyl chain.
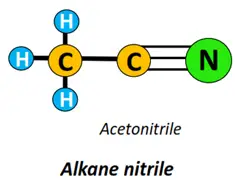
- Nitrile (-CN) functional group: Alkane nitriles (R-CN) are formed if the alkyl group carries a C-atom, triple-covalently bonded to a nitrogen atom.
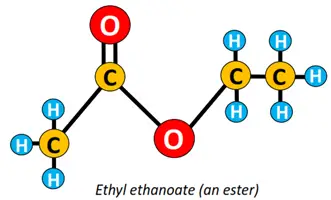
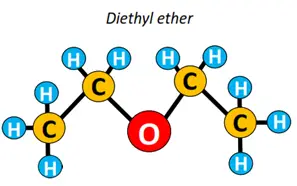
Other functional groups attached to the alkyl chain include amine (-NH2), anhydride (-CO)2O, ester (-COOR), ether (C-O-C), etc.
Nomenclature in organic chemistry
The name of any organic compound usually consists of a root word derived from a Greek or Latin numeral indicating the number of C-atoms present in it. The second part (suffix) added to the root word comes from the functional group attached.
Root words for ‘’alk’ include;
- Meth: 1 carbon atom
- Eth: 2 carbon atoms
- Prop: 3 carbon atoms
- But: 4 carbon atoms
- Pent: 5 carbon atoms
- Hex: 6 carbon atoms
- Hept: 7 carbon atoms
So on and so forth!
With alk, the suffix added as per the functional group is:
- ane: for alkanes
- ene: for alkenes
- yne: for alkynes
- ol: for alcohol
- al: for aldehyde
- one: for ketones
- oic: for carboxylic acids
Examples:
If an alkane molecule consists of 2 carbon atoms, then eth is used with the suffix ane. This gives the name ethane.
Removing e from ethane and adding ol gives us ethanol, i.e., an alcohol molecule containing 2 C-atoms and an OH group.
Adding al next to ethan gives us ethanal (another name for acetaldehyde).
Similarly, oic used as a suffix to ethan forms ethanoic acid (more commonly known as acetic acid).
The condensation of an ethanol molecule with ethanoic acid yields ethyl ethanoate (an ester). Conversely, ethyl propanoate is an ester formed by the chemical reaction between propanol and ethanoic acid, which implies that the alkyl part in the name of an ester comes from carboxylic acid, while the alkanoate is derived from the corresponding alcohol.
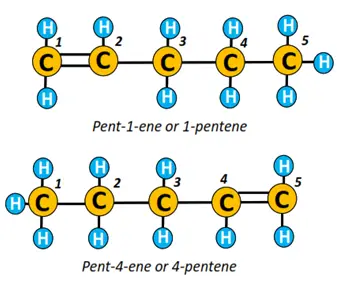
Pentene is an alkene molecule comprising 5 C-atoms and a double covalent bond. The chain is numbered from 1 to 5, starting from the left or right side.
If a C=C bond is present between C1 and C2 (as shown below), it is known as pent-1-ene. Conversely, if it is located between C4 and C5, then it is called the alk-lowest carbon number carrying double bond-ene, i.e., pent-4-ene in this case.
If a carbon chain contains a specific functional group such as OH, COOH or C=O, then the position of the functional group strongly influences its IUPAC name.
In the structure drawn below, the carbon chain is numbered from 1 to 3; number 1 is assigned to the C-atom nearest to the OH group. Its name is written as: alk-carbon number carrying OH group-ol, i.e., propan-1-ol.
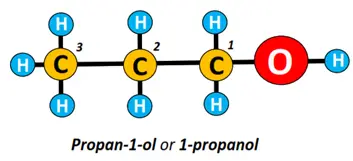
Can you name the isomer of propan-1-ol given below using the above technique?
Well, the name of the above organic structure is propan-2-ol because the OH group is present at carbon number 2.
Different types of reactions in organic chemistry
The chemical reactions of organic compounds are primarily divided into the following main types:
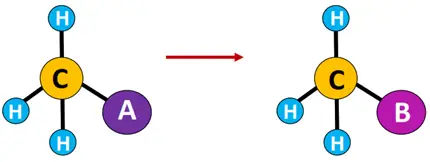
Substitution reaction: A reaction in which the functional group or an atom of the reacting organic compound (substrate) is replaced by another group or atom of the reagent.
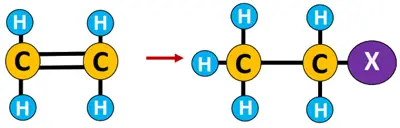
Elimination reaction: A reaction in which one or two molecules are expelled from the substrate, resulting in multiple covalent bonds or ringed structures.

Addition reaction: A reaction in which a ringed structure or multiple bonds breaks down to accommodate one or two molecules from the reagent. It is the opposite of an elimination reaction.
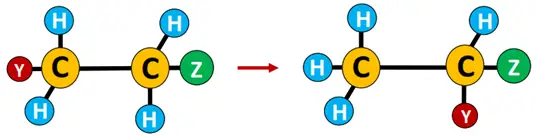
Rearrangement reaction: Reactions involving a change in bond connectivity within the organic molecule are known as rearrangements. It involves the migration of an atom or a functional group from one location to another within the molecule.
Learn all about the above organic chemistry reactions in a subsequent article.
Here is for you an organic chemistry reactions’ cheat sheet so that in your next chemistry class, you know it all!
Why is organic chemistry important?
Organic chemistry is important because:
- It supports all life forms on Earth. Although it is not the most abundant natural element, still all living organisms are chiefly composed of organic compounds, aside from water. These include carbohydrates, fatty acids, cholesterol, amino acids, proteins, nucleic acids (DNA), and many more.
- Organic compounds are naturally found in food items such as capsaicin (present in chilli peppers), hesperidin (in grapefruit, oranges, lemon and lime), beta-carotene (in carrots), caffeine (in tea and coffee), etc. Studying organic chemistry is thus important in order to understand the chemical composition of what we eat and what benefits they give us.
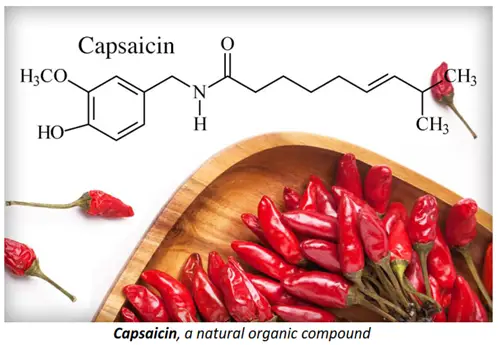
- Organic chemistry is fundamentally important in medicine and biotechnology. It includes the synthesis and study of pharmaceutical drugs, steroids, therapeutic agents, artificial implants, growth hormones, etc.
- Plastic, paint, dyes, ink, paper, rubber tires, etc., are all synthetic organic compounds. Thus, organic chemistry is also important for advancement and product synthesis in industry and in research and development (R & D).
- A thorough understanding of organic chemistry has enabled scientists to synthesize new compounds and molecules using renewable resources. This saved the world from the depletion of non-renewable resources such as coal and petroleum.
Find other specific applications of organic chemistry here.
Are all carbon-containing compounds organic?
You must note that all organic compounds contain carbon as an essential element. However, not all C-containing compounds are organic in nature. For instance, diamond, graphite, and buckminsterfullerene are all hot sensations of the 20th century. These are allotropes of carbon, but they are not essentially organic in nature because these consist of only one type of atom, i.e., carbon.
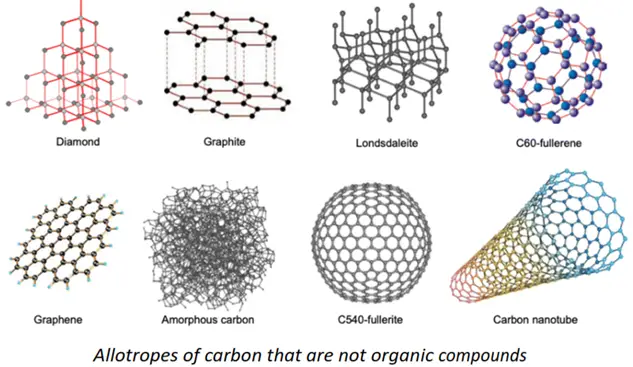
‘For an organic compound, the carbon atoms must be bonded to other atoms in addition to being self-linked”.
You may also like our special article on organic spectroscopy! Happy learning.
References
1. H. Brown, William, and Thomas Poon. 2016. Introduction to Organic Chemistry, 6th Edition.
2. Morrison, Robert Thornton, Robert Neilson Boyd, and Saibal Kanti Bhattacharjee. 2013. Organic Chemistry, 7th Edition.
3. Patrick, Graham. 2017. Organic Chemistry: A Very Short Introduction (Oxford University Press).
